- Propylene carbonate
-
Propylene carbonate[1] 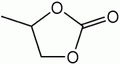
 4-Methyl-1,3-dioxolan-2-oneOther namesCyclic propylene carbonate
4-Methyl-1,3-dioxolan-2-oneOther namesCyclic propylene carbonate
Carbonic acid propylene ester
Cyclic 1,2-propylene carbonate
Propylene glycol cyclic carbonate
1,2-Propanediol carbonate
4-Methyl-2-oxo-1,3-dioxolane
Arconate 5000
Texacar PCIdentifiers CAS number 108-32-7 
PubChem 7924 ChemSpider 10609770 
UNII 8D08K3S51E 
Jmol-3D images Image 1 - OC(=O)OCC(C)OC(=O)[O-]
Properties Molecular formula C4H6O3 Molar mass 102.09 g/mol Appearance Colorless liquid Density 1.205 g/cm3 Melting point -55 °C, 218 K, -67 °F
Boiling point 240 °C, 513 K, 464 °F
Solubility in water Very soluble [2] Refractive index (nD) 1.4189 [3] Hazards MSDS MSDS by Mallinckrodt Baker R-phrases R36 S-phrases S26 S36 Main hazards Xi NFPA 704 Flash point 132 °C Autoignition
temperature455 °C  carbonate (verify) (what is:
carbonate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Propylene carbonate (often abbreviated PC) is an organic compound, a cyclic carbonate of propylene glycol.[4] This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate exists as two enantiomers but is often used as the racemic mixture.[5]
Contents
Preparation
Propylene carbonate is a byproduct of the synthesis of polypropylene carbonate from propylene oxide and carbon dioxide. It can be intentionally manufactured from the same feedstocks, using different reaction conditions. It can be also synthesized from, e.g., urea and propylene glycol over zinc-iron double oxide catalyst.
Applications
Propylene carbonate is used in a variety of syntheses and applications as a polar, aprotic solvent. It has a high molecular dipole moment (4.9 D), considerably higher that those of acetone (2.91 D) and ethyl acetate (1.78 D).[6] It is possible, for example, to obtain potassium, sodium, and other alkali metals by electrolysis of their chlorides and other salts dissolved in propylene carbonate.[7]
Due to its high dielectric constant of 64, it is frequently used as a high-permittivity component of electrolytes in lithium batteries, usually together with a low-viscosity solvent (e.g. dimethoxyethane). Its high polarity allows it to create an effective solvation shell around lithium ions, thereby creating a conductive electrolyte. However, it is not used in lithium-ion batteries due to its destructive effect on graphite.[8]
Propylene carbonate can also be found in some adhesives, paint strippers, and in some cosmetics.[9] It is also used as plasticizer.
See also
References
- ^ Propylene carbonate at Sigma-Aldrich
- ^ CRC Handbook of Chemistry and Physics - Student Edition. 76th Edition ed, ed. D.R. Lide. 1995: CRC Press.
- ^ CRC Handbook of Chemistry and Physics - Student Edition. 76th Edition ed, ed. D.R. Lide. 1995: CRC Press.
- ^ WebBook page for propylene carbonate
- ^ Dieter Stoye “Solvents” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Wienheim. doi:10.1002/14356007.a24_437
- ^ CRC Handbook of Chemistry and Physics, 91st Ed. 2010-2011. <http://www.hcpnetbase.com>.
- ^ J. Jorné and C. W. Tobias (1975), Electrodeposition of the alkali metals from propylene carbonate. Journal of Applied Electrochemistry, volume 5 issue 4, pp. 279-290. doi:10.1007/BF00608791
- ^ Doron Aurbach (1999), "Nonaqueous Electrochemistry", Marcel Dekker, Inc.
- ^ Record in the Household Products Database of NLM
Categories:- Ester solvents
- Plasticizers
- Carbonate esters
Wikimedia Foundation. 2010.

