- Disodium aurothiomalate
-
Disodium aurothiomalate 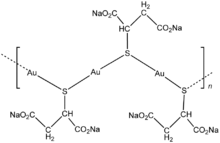
Clinical data Pregnancy cat. ? Legal status ? Routes Intramuscular injection Pharmacokinetic data Bioavailability 0% Excretion Renal, very slow Identifiers CAS number 12244-57-4 ATC code None PubChem CID 71443 Chemical data Formula C4H3AuNa2O4S Mol. mass 390.076 g/mol  (what is this?) (verify)
(what is this?) (verify)Disodium aurothiomalate is a chemical compound with the formula AuSCH(CO2Na)CH2CO2Na. In conjunction with its monoprotonated derivative, this coordination complex or closely related species are used to treat rheumatoid arthritis, under the tradename Myochrysine.[1] The thiomalate is racemic in most formulation.
Contents
Structure
Disodium aurothiomalate is a coordination polymer. The salt CsNa2Au2T(TH) salt (T = thiomalate3-, TH = monoprotonated thiomalate2-) is related to disodium aurothiomalate but is easier to crystallise and characterise by X-ray crystallography. The compound is polymeric with Au-S-Au-S... chains with succinoyl groups attached to the sulfur atoms.[2] The structure of the related drug Aurothioglucose is also polymeric with two-coordinate gold(I) centers.[1] In such compounds, the efficacy results from the compound in solution, the structures of such solution species are often poorly understood. Medical texts sometimes suggest that free Au+ ions exist in this and related gold(I) compounds, but the Au-thiolate bonding is highly covalent and free gold ions do not exist in solution. Whereas simple gold thiolates are lipophilic, the carboxylate substituents render disodium aurothiomalate soluble in water.
Disodium aurothiomalate contains no Au-C bonds, so it is not an organometallic compound in the formal sense.
Mechanism of action
Unknown. In vitro experiments have shown that Au(I) can inhibit lymphocyte proliferation, lysosomal enzyme release, the release of reactive oxygen species from macrophages, and IL-1 production.
Gold salts can decrease the inflammation of the joint lining. This effect can prevent destruction of bone and cartilage. In former times gold salts were used as second-line drugs (used when the arthritis progressed in spite of antiinflammatory drugs). Nowadays such former "second-line" drugs are used from the beginning of therapy to inhibit joint erosion if possible.
Side effects
Disodium aurothiomalate can cause photosensitive rashes, gastrointestinal disturbance, and kidney damage.
See also
References
- ^ a b Shaw, III, C. F. (1999). "Gold-Based Therapeutic Agents". Chemical Reviews 99 (9): 2589–600. doi:10.1021/cr980431o. PMID 11749494.
- ^ Bau, R. (1998). "Crystal Structure of the Antiarthritic Drug Gold Thiomalate (Myochrysine): A Double-Helical Geometry in the Solid State". Journal of the American Chemical Society 120 (36): 9380–1. doi:10.1021/ja9819763.
Specific antirheumatic products / DMARDs (M01C) Quinolines Gold preparations Other Penicillamine #/Bucillamine • Chloroquine #/Hydroxychloroquine • Leflunomide • Sulfasalazine # • antifolate (Methotrexate #) • thiopurine (Azathioprine) #M: JNT
anat(h/c, u, t, l)/phys
noco(arth/defr/back/soft)/cong, sysi/epon, injr
proc, drug(M01C, M4)
Categories:- Gold compounds
- Immunosuppressants
Wikimedia Foundation. 2010.
