- Cinnoline
-
Cinnoline  CinnolineOther namesBenzopyridazine
CinnolineOther namesBenzopyridazineIdentifiers CAS number 253-66-7 PubChem 9208 ChemSpider 8853 
UNII N5KD6I506O 
ChEBI CHEBI:36617 
ChEMBL CHEMBL479792 
Jmol-3D images Image 1 - n1nccc2ccccc12
Properties Molecular formula C8H6N2 Molar mass 130.15 g/mol Melting point 39 °C, 312 K, 102 °F
Acidity (pKa) 2.64[1]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with quinoxaline, phthalazine and quinazoline.
Contents
Properties
The free base can be obtained as an oil by treatment of the hydrochloride with base. It co-crystallizes with one molecule of ether as white silky needles, (m.p. 24-25 °C) upon cooling ethereal solutions. The free base melts at 39 °C. It has a taste resembling that of chloral hydrate and leaves a sharp irritation for some time. Cinnoline derivatives are obtained from oxycinnolin carboxylic acid, which is formed by digesting orthophenyl propiolic acid diazo chloride with water. Oxycinnolin carboxylic acid on heating gives oxycinnoline, melting at 225 °C, which with phosphorus pentachloride gives chlorcinnolin. This substance is reduced by iron filings and sulfuric acid to dihydrocinnolin.
Discovery and synthesis
The compound was first obtained in impure form by cyclization of the alkyne o-C6H4(NH2)C≡CCO2H in water to give 4-hydroxycinnoline-3-carboxylic acid. This material could be decarboxylated and the hydroxyl group reductively removed to give the parent heterocycle. This reaction is called the Richter cinnoline synthesis[2] Improved methods exist for its synthesis. It can be prepared by dehydrogenation of dihydrocinnoline with freshly precipitated mercuric oxide. It can be isolated as the hydrochloride.[3]
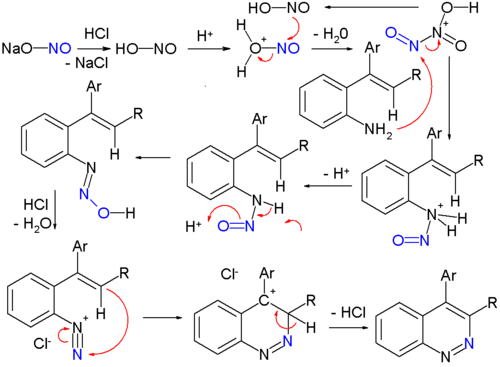
Cinnolines are cinnoline derivatives. A classic organic reaction for synthesizing cinnolines is the Widman-Stoermer synthesis[4], a ring-closing reaction of an α-vinyl- aniline with hydrochloric acid and sodium nitrite:
The sodium nitrite is first converted to nitrous acid which then forms the electrophilic intermediate dinitrogen trioxide. The next intermediate is the stable Nitrosamine with goes on to lose water forming the diazonium salt which then reacts with the vinyl group in the ring-closing step. A conceptually related reaction is the Bamberger triazine synthesis towards triazines.
Another cinnoline method is the Borsche cinnoline synthesis.
Safety
Cinnoline is toxic.[citation needed]
See also
References
- ^ Brown, H.C., et al., in Baude, E.A. and Nachod, F.C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- ^ Victor von Richter "Über Cinnolinderivate" Chemische Berichte, 1883, volume 16, pp 677-683.
- ^ Parrick, J.; Shaw, C. J. G. and Mehta, L. K., "Pyridazines, cinnolines, benzocinnolines and phthalazines", Rodd's Chemistry of Carbon Compounds (2nd Edition), 2000, 4, 1-69.
- ^ Name Reactions and Reagents in Organic Synthesis Bradford P. Mundy, Michael G. Ellerd, Frank G. Jr. Favaloro 2005 ISBN 0471228540
Categories:- Aromatic bases
- Cinnolines
Wikimedia Foundation. 2010.