- Sodium bis(trimethylsilyl)amide
-
Sodium bis(trimethylsilyl)amide 
 Sodium bis(trimethylsilyl)amideSystematic nameSodiobis(trimethylsilyl)amineOther namesSodium hexamethyldisilazane
Sodium bis(trimethylsilyl)amideSystematic nameSodiobis(trimethylsilyl)amineOther namesSodium hexamethyldisilazane
Sodium hexamethyldisilazideIdentifiers Abbreviations NaHMDS CAS number 1070-89-9 PubChem 2724254 
ChemSpider 21169873 
EC number 213-983-8 UN number UN 3263 Beilstein Reference 3629917 Jmol-3D images Image 1 - C[Si](C)(C)N([Na])[Si](C)(C)C
- InChI=1S/C6H18NSi2.Na/c1-8(2,3)7-
9(4,5)6;/h1-6H3;/q-1;+1
Key: WRIKHQLVHPKCJU-UHFFFAOYSA-N
InChI=1/C6H18NSi2.Na/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1/rC6H18NNaSi2/c1-9(2,3)7(8)10(4,5)6/h1-6H3
Key: WRIKHQLVHPKCJU-JSJAVMDOAQ
InChI=1S/C6H18NSi2.Na/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1
Key: WRIKHQLVHPKCJU-UHFFFAOYSA-N
Properties Molecular formula C6H18NNaSi2 Molar mass 183.37 g/mol Appearance off-white solid Density 0.9 g/cm3, solid Melting point 171-175 °C, 444-448 K, 340-347 °F
Boiling point 170 °C, 443 K, 338 °F (2 mmHg)
Solubility in water reacts with water Solubility in other solvents THF, benzene
tolueneStructure Molecular shape Triangular pyramidal Hazards R-phrases R11 R15 R34 S-phrases S16 S24/25 Main hazards Highly flammable, corrosive Related compounds Other cations Lithium
bis(trimethylsilyl)amide
(LiHMDS)
Potassium
bis(trimethylsilyl)amideRelated compounds Lithium diisopropylamide (LDA)
KHExcept where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Sodium bis(trimethylsilyl)amide is the chemical compound with the formula ((CH3)3Si)2NNa. This species, usually called NaHMDS (sodium hexamethyldisilazide), is a strong base used for deprotonation reactions or base catalyzed reaction. Its advantages are that it is available as a solid and it is soluble in a wide range of nonpolar solvents such as THF, diethyl ether, benzene, and toluene by virtue of the lipophilic TMS groups.[1]
NaHMDS is quickly destroyed by water to form sodium hydroxide and bis(trimethylsilyl)amine.
Contents
Structure
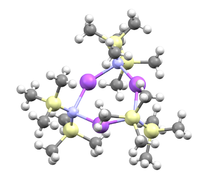
It is common that polar organometallic reagents are depicted as ions, when in fact such species are rarely ionic. The structure shown in the figure is a better representation - the sodium atom is attached to the nitrogen atom via a polar covalent bond. When unsolvated, this compound is trimeric in the solid state.[2]
Applications in synthesis
NaHMDS is widely used as a base for C-H acids. Typical reactions:
- To deprotonate ketones and esters to generate enolate derivatives.
- Generate halocarbenes such as CHBr and CHI by dehydrohalogenation of the CH2X2 (X = Br, I). These carbene reagents add to alkenes to give substituted cyclopropanes.
- To generate Wittig reagents via deprotonation of phosphonium salts.
- Deprotonate cyanohydrins.
NaHMDS is also used as a base for N-H acids.
NaHMDS reacts with alkyl halides to give amine derivatives:
- (CH3)3Si)2NNa + RBr → (CH3)3Si)2NR + NaBr
- (CH3)3Si)2NR + H2O → (CH3)3Si)2O + RNH2
This method has been extended to aminomethylation via the reagent (CH3)3Si)2NCH2OMe, which contains a displaceable methoxy group.
- To deprotonate precursors to give stable carbenes.
See also
References
- ^ Watson, B. T.; Lebel, H. "Sodium bis(trimethylsilyl)amide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rs071m.pub2
- ^ Driess, Matthias; Pritzkow, Hans; Skipinski, Markus; Winkler, Uwe (1997). "Synthesis and Solid State Structures of Sterically Congested Sodium and Cesium Silyl(fluorosilyl)phosphanide Aggregates and Structural Characterization of the Trimeric Sodium Bis(trimethylsilyl)amide". Organometallics 16 (23): 5108–5112. doi:10.1021/om970444c.
Categories:- Organosilicon compounds
- Bis(trimethylsilyl)amides
- Sodium compounds
Wikimedia Foundation. 2010.
