- Phenylacetone
-
Phenylacetone 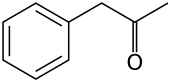
 1-phenylpropan-2-oneOther namesbenzyl methyl ketone; methyl benzyl ketone; phenyl-2-propanone
1-phenylpropan-2-oneOther namesbenzyl methyl ketone; methyl benzyl ketone; phenyl-2-propanoneIdentifiers CAS number 103-79-7 
ChemSpider 21106366 
UNII O7IZH10V9Y 
KEGG C15512 
ChEBI CHEBI:52052 
Jmol-3D images Image 1 - O=C(C)Cc1ccccc1
Properties Molecular formula C9H10O Molar mass 134.18 g mol−1 Density 1.006 g/mL Melting point -15 °C
Boiling point 214 — 216 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phenylacetone (known also as phenyl-2-propanone, benzyl methyl ketone, or methyl benzyl ketone), is an organic compound. It is a clear oil with a refractive index of 1.5168. This substance is used in the manufacture of methamphetamine and amphetamine as a starting material or intermediate, where it is commonly known as P2P. Due to the illicit uses in clandestine chemistry, it was declared a schedule II controlled substance in the United States 11. February 1980.[1]
Preparation
There are many methods in the scientific literature to prepare phenylacetone, and due to its status as a controlled substance, there is crossover into popular literature such as works by Uncle Fester and Alexander Shulgin. Large amounts of data are available on the Internet relating to the preparation of phenylacetone.
A conceptually simple example of phenylacetone organic synthesis is the Friedel-Crafts alkylation of benzene with chloroacetone.
Phenylacetone can also be produced from many other compounds. For example:
- phenylacetic acid is distilled with lead acetate or calcium acetate to yield phenylacetone.
- benzaldehyde is reacted with nitroethane yielding phenyl-2-nitropropene, which is reduced, usually in the presence of acid, to phenylacetone.
Phenyl acetone is used as an intermediate to produce pesticides and anticoagulants. Active ingredients as anticoagulant include:- Brodifacoum
- Chlorophacinone
- Coumachlor
- Difenacoum
- Diphacinane
- 2-Pivaloyl-1,3-indandione
- 2-Isovaleryl-1,3-indandione
- Warfarin
See also
References
Categories:- Ketones
- Phenyl compounds
Wikimedia Foundation. 2010.

