- tert-Butyl chloride
-
tert-Butyl chloride 
 2-chloro-2-methylpropaneOther names1,1-dimethylethyl chloride
2-chloro-2-methylpropaneOther names1,1-dimethylethyl chloride
1-chloro-1,1-dimethylethane
chlorotrimethylmethane
trimethylchloromethane
t-butyl chlorideIdentifiers CAS number 507-20-0 
PubChem 10486 ChemSpider 10054 
EC-number 208-066-4 UN number 1127 ChEMBL CHEMBL346997 
RTECS number TX5040000 Jmol-3D images Image 1 - ClC(C)(C)C
Properties Molecular formula C4H9Cl Molar mass 92.57 g/mol Appearance Colorless liquid Density 0.84 g cm−3 Melting point −26 °C, 247 K, -15 °F
Boiling point 51 °C, 324 K, 124 °F
Solubility in water Sparingly sol in water, miscible with alcohol and ether Vapor pressure 34.9 kPa (20 °C) Hazards EU classification Flammable (F) R-phrases R12, R36/37/38 S-phrases S7, S9, S16, S29, S33 NFPA 704 Flash point −9 °C (open cup)
−23 °C (closed cup)Autoignition
temperature540 °C Related compounds Related alkyl halides tert-Butyl bromide  chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references tert-Butyl chloride is a colorless, liquid organic compound at room temperature. It is sparingly soluble in water, with a tendency to undergo spontaneous solvolysis when dissolved into it. The compound is flammable and volatile, and its main use is as a starting molecule to carry out nucleophilic substitution reactions, to produce different substances, ranging from alcohols to alkoxide salts.
When tert-butyl chloride is dissolved in water, a polar and protic solvent, the bulky chloride substituent is carried away by it, and isolated from the aliphatic chain, causing an heterolytic rupture of the compound, giving rise to a carbocation which eventually becomes a tertiary alcohol after a water molecule reacts with it, releasing hydrochloric acid as the final product. If a different, stronger nucleophilic agent is present at the moment of reaction, reaction product may not be an alcohol, but a tertiary carbon with the nucleophile as a substituent.
Synthesis
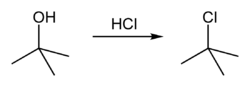
tert-Butyl chloride can be synthesized in the laboratory by the SN1 reaction of tert-Butanol with concentrated hydrochloric acid, as shown below.
Step 1 Step 2 Step 3 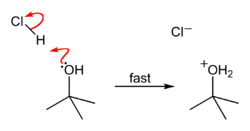
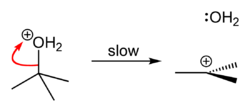
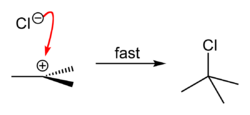
The acid protonates the alcohol, forming a good leaving group (water). Water leaves the protonated t-BuOH, forming a relatively stable tertiary carbocation. The chloride ion attacks the carbocation, forming t-BuCl. The overall reaction, therefore, is:
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
See also
External links
Categories:- Organochlorides
Wikimedia Foundation. 2010.