- alpha-Viniferin
-
α-Viniferin 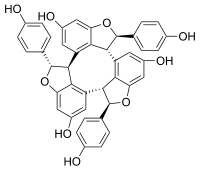 Other namesα-Viniferin; (+)-α-Viniferin
Other namesα-Viniferin; (+)-α-ViniferinIdentifiers CAS number 62218-13-7 
PubChem 196402 ChemSpider 333272 
ChEMBL CHEMBL443463 
Jmol-3D images Image 1
Image 2- OC(C=C1)=CC=C1[C@@H]2[C@@H](C3=C4C(O[C@@H](C5=CC=C(O)C=C5)[C@@H]4C6=C([C@@H]7[C@H](C8=CC=C(O)C=C8)O9)C9=CC(O)=C6)=CC(O)=C3)C%10=C7C=C(O)C=C%10O2
Oc1ccc(cc1)C%10Oc2cc(O)cc7c2C%10c8cc(O)cc9OC(c3ccc(O)cc3)C(c5cc(O)cc6OC(c4ccc(O)cc4)C7c56)c89
- InChI=1S/C42H30O9/c43-22-7-1-19(2-8-22)40-37-28-13-25(46)17-32-35(28)39(42(50-32)21-5-11-24(45)12-6-21)30-15-27(48)18-33-36(30)38(29-14-26(47)16-31(49-40)34(29)37)41(51-33)20-3-9-23(44)10-4-20/h1-18,37-48H

Key: KUTVNHOAKHJJFL-UHFFFAOYSA-N
InChI=1/C42H30O9/c43-22-7-1-19(2-8-22)40-37-28-13-25(46)17-32-35(28)39(42(50-32)21-5-11-24(45)12-6-21)30-15-27(48)18-33-36(30)38(29-14-26(47)16-31(49-40)34(29)37)41(51-33)20-3-9-23(44)10-4-20/h1-18,37-48H
Key: KUTVNHOAKHJJFL-UHFFFAOYAE
Properties Molecular formula C42H30O9 Molar mass 678.68 g mol−1 Exact mass 678.188983 u  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references α-Viniferin is a stilbene trimer. It can be isolated from Caragana chamlagu[1] and from Caragana sinica[2]
It has been shown to inhibit acetylcholinesterase.[1]
References
- ^ a b Sung, Sang Hyun; Kang, So Young; Lee, Ki Yong; Park, Mi Jung; Kim, Jeong Hun; Park, Jong Hee; Kim, Young Chul; Kim, Jinwoong et al. (2002). "(+)-α-Viniferin, a Stilbene Trimer from Caragana chamlague, Inhibits Acetylcholinesterase". Biological & Pharmaceutical Bulletin 25: 125. doi:10.1248/bpb.25.125.
- ^ Shu, N; Zhou, H; Hu, C (2006). "Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method". Biological & pharmaceutical bulletin 29 (4): 608–12. doi:10.1248/bpb.29.608. PMID 16595888.
Stilbenoids Combretastatin | Combretastatin A-1 | Combretastatin A-4 | Combretastatin B-1 | Piceatannol | Pinosylvin | Pterostilbene | ResveratrolGlycosides Astringin | Piceid | RhaponticinOligomeric forms Ampelopsin A | Ampelopsin E | Carasinol B | Diptoindonesin C | Diptoindonesin F | Flexuosol A | Gnetin H | Hemsleyanol D | Kobophenol A | Laetevirenol A, B, C, D and E | Vaticanol B | Viniferal | Tetramers : Laetevirenol F and GOligomers of resveratrolDimers : ε-viniferin, Parthenocissin A, Quadrangularin A, Pallidol and Amurensin A | Trimers : α-viniferin, Laevifonol (an ε-viniferin-ascorbic acid hybrid compound), trans-diptoindonesin B | Tetramers : Hopeaphenol | Vitisins A, B and COligomeric forms glycosides Diptoindonesin A | Laevifoside (O-glucoside of ampelopsin A)Misc Hydroxystilbamidine | Alpha-Phenylcinnamic acidSynthetic Stilbenoid drugsThis article about a natural phenol is a stub. You can help Wikipedia by expanding it. - OC(C=C1)=CC=C1[C@@H]2[C@@H](C3=C4C(O[C@@H](C5=CC=C(O)C=C5)[C@@H]4C6=C([C@@H]7[C@H](C8=CC=C(O)C=C8)O9)C9=CC(O)=C6)=CC(O)=C3)C%10=C7C=C(O)C=C%10O2
