- Beryllium oxide
-
Beryllium oxide 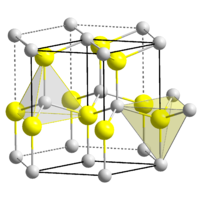 Beryllium oxide[citation needed]Systematic nameOxoberyllium[1]Other namesBerlox[citation needed]
Beryllium oxide[citation needed]Systematic nameOxoberyllium[1]Other namesBerlox[citation needed]
Beryllia[citation needed]
Thermalox[citation needed]
Beryllia ceramic[citation needed]
Super beryllia[citation needed]Identifiers CAS number 1304-56-9 
PubChem 14775 ChemSpider 14092 
EC number 215-133-1 UN number 1566 MeSH beryllium+oxide ChEBI CHEBI:62842 
RTECS number DS4025000 Beilstein Reference 3902801 Jmol-3D images Image 1
Image 2- [Be]=[O]
[Be-]#[O+]
Properties Molecular formula BeO Molar mass 25.01 g mol−1 Exact mass 25.007096757 g mol−1 Appearance Colourless, vitreous crystals Odor Odourless Density 3.01 g cm−3 Melting point 2507 °C, 2780 K, 4545 °F
Boiling point 3900 °C, 4173 K, 7052 °F
Band gap 10.6 eV Thermal conductivity 330 W K−1 m−1 Refractive index (nD) 1.7 Structure Crystal structure Hexagonal Space group P63mc Point group C6v Coordination
geometryTetragonal Molecular shape Linear Thermochemistry Std enthalpy of
formation ΔfHo298–611.8—607.0 kJ mol−1 Standard molar
entropy So29813.73–13.81 J K−1 mol−1 Hazards MSDS External MSDS GHS pictograms 

GHS signal word DANGER GHS hazard statements H301, H315, H317, H319, H330, H335, H350, H372 GHS precautionary statements P201, P260, P280, P284, P301+310, P305+351+338 EU Index 004-003-00-8 EU classification  T+
T+R-phrases R49, R25, R26, R36/37/38, R43, R48/23 S-phrases S53, S45 NFPA 704 LD50 2.062 g kg−1 (mouse, oral) Related compounds Other anions Beryllium telluride Other cations Magnesium oxide
Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and actually exceeds that of some metals.[2] As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory.[3] It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide.
Contents
Preparation and chemical properties
Beryllium oxide can be prepared by calcining (roasting) beryllium carbonate, dehydrating beryllium hydroxide or igniting the metal:
- BeCO3→ BeO + CO2
- Be(OH)2 → BeO + H2O
- 2 Be + O2 → 2 BeO
Igniting beryllium in air gives a mixture of BeO and the nitride Be3N2.[2] Unlike oxides formed by the other group 2 (alkaline earth metals), beryllium oxide is amphoteric rather than basic.
Beryllium oxide formed at high temperatures (>800°C) is inert, but dissolves easily in hot aqueous ammonium bifluoride (NH4HF2) or a hot solution of concentrated sulfuric acid (H2SO4) and ammonium sulfate ((NH4)2SO4).
Structure
BeO crystallizes in the hexagonal wurtzite structure, featuring tetrahedral Be2+ and O2- centres. In contrast, the oxides of the larger group 2 metals, i.e., MgO, CaO, SrO, BaO, crystallize in the cubic rock salt motif with octahedral geometry about the dications and dianions.[2] At high temperature the structure transforms to a tetragonal form.[4] In the vapor phase, beryllium oxide is present as discrete diatomic molecules. In the language of valence bond theory, diatomic BeO can be described as adopting sp orbital hybridisation, featuring one sigma and two pi bonds, by the overlap of the s and pz orbitals, and px and py of both atoms. According to the Aufbau principle, two electrons occupy the degenerate pi orbitals (px and py), giving a total bond order of three. Two electons are unpaired, consistent with the paramagnetism of diatomic BeO.
Applications
High quality crystals may be grown hydrothermally, or otherwise by the Verneuil method. For the most part, beryllium oxide is produced as a white amorphous powder, sintered into larger shapes. Impurities like carbon, trapped with the crystals can give a variety of colours to the otherwise colourless host crystals.
Sintered beryllium oxide, which is very stable, has ceramic characteristics.[5] Beryllium oxide is used in rocket engines.
Beryllium oxide is used in many high-performance semiconductor parts for applications such as radio equipment because it has good thermal conductivity while also being a good electrical insulator. It is used as a filler in some thermal interface materials such as thermal grease.[6] Some power semiconductor devices have used beryllium oxide ceramic between the silicon chip and the metal mounting base of the package in order to achieve a lower value of thermal resistance than for a similar construction made with aluminium oxide. It is also used as a structural ceramic for high-performance microwave devices, vacuum tubes, magnetrons, and gas lasers.
Safety
Like all beryllium compounds, BeO is carcinogenic and may cause chronic beryllium disease. Once fired into solid form, it is safe to handle as long as it is not subjected to any machining that generates dust.[7] Beryllium oxide ceramic is not a hazardous waste under Federal law in the USA.
References
- ^ "beryllium oxide – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related records. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=14775&loc=ec_rcs. Retrieved 8 November 2011.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- ^ Raymond Aurelius Higgins (2006). Materials for Engineers and Technicians. Newnes. p. 301. ISBN 0750668504. http://books.google.com/?id=6TKSG6LWIEQC&pg=PA301.
- ^ A.F. Wells (1984). Structural Inorganic Chemistry (5 ed.). Oxford Science Publications. ISBN 0-19-855370-6.
- ^ Günter Petzow, Fritz Aldinger, Sigurd Jönsson, Peter Welge, Vera van Kampen, Thomas Mensing, Thomas Brüning"Beryllium and Beryllium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_011.pub2
- ^ Greg Becker, Chris Lee, and Zuchen Lin (2005). "Thermal conductivity in advanced chips — Emerging generation of thermal greases offers advantages". Advanced Packaging: 2–4. http://www.apmag.com/. Retrieved 2008-03-04.
- ^ Beryllium Oxide Safety
External links
- Beryllium Oxide MSDS from American Beryllia
- Beryllium Oxide Properties (solid form)
- IARC Monograph "Beryllium and Beryllium Compounds"
- International Chemical Safety Card 1325
- National Pollutant Inventory – Beryllium and compounds
- NIOSH Pocket guide to Chemical Hazards
Beryllium compounds Categories:- Beryllium compounds
- Oxides
- IARC Group 1 carcinogens
- Ceramic materials
- [Be]=[O]
Wikimedia Foundation. 2010.


