- Dimethyl fumarate
-
Dimethyl fumarate 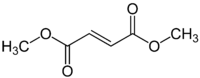 Dimethyl (E)-butenedioateOther namestrans-1,2-Ethylenedicarboxylic acid dimethyl ester
Dimethyl (E)-butenedioateOther namestrans-1,2-Ethylenedicarboxylic acid dimethyl ester
(E)-2-Butenedioic acid dimethyl esterIdentifiers CAS number 624-49-7 
ChemSpider 553171 
UNII FO2303MNI2 
EC number 210-849-0 Jmol-3D images Image 1 - O=C(OC)\C=C\C(=O)OC
Properties Molecular formula C6H8O4 Molar mass 144.127 g/mol Appearance White crystalline solid Density 1.37 g/cm³, solid Melting point 102-105 °C[1]
Boiling point 192-193 °C[1]
Hazards EU classification Harmful (Xn) R-phrases R21 R38 R41 R43 S-phrases S26 S36 S37 S39 Related compounds Related diesters Diethyl fumarate, dimethyl maleate, dimethyl malonate, dimethyl adipate Related compounds Fumaric acid
Methyl acrylate fumarate (verify) (what is:
fumarate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethyl fumarate is the methyl ester of fumaric acid.
Contents
Reactions
Dimethyl fumarate is an ester and an α,β-unsaturated electrophilic compound, undergoing reactions typical to them. It is also a diene acceptor in the ordinary Diels-Alder reaction, where the reactivity of its vinylidenic bond is enchanced by the two electron-withdrawing ester groups. Due to the geometry of the starting ester, the Diels-Alder product will have a trans configuration.
Uses
Dimethyl fumarate is used to treat psoriasis.[2] It is a lipophilic, highly mobile molecule in human tissue. However, as an α,β-unsaturated ester, dimethyl fumarate reacts rapidly with the detoxifying agent glutathione by Michael addition. When administered orally, it does not survive long enough to be absorbed into blood.[3]
Another use for dimethyl fumarate is mold inhibition, mostly for leather products. But see below for the problems this has caused.
Tests in mouse models have been conducted with the aim of using it as part of a specific cancer treatment.[4][5]
Dimethyl fumarate has been shown to provide benefit in a mouse model of multiple sclerosis and phase III clinical trials in patients are in progress [6][7] As BG-12 it has shown good results with relapsing-remitting multiple sclerosis.[8]
Risks
Dimethyl fumarate has been found to be an allergic sensitizer at very low concentrations, producing extensive, pronounced eczema that is difficult to treat. Concentrations as low as 1 ppm may produce allergic reactions.[9] There are only a handful of equally potent sensitizers.[10]
The extreme sensitizing risk was brought to public attention by the "poison chair" incident, where Chinese manufacturer Linkwise produced two-seater sofas with dimethyl fumarate sachets inside to inhibit mould while they were in storage or transport.[11][12] In Finland where the chairs were sold from 2006–2007, sixty users were given serious rashes.[10] The cause was identified as dimethyl fumarate-induced allergic reaction by Tapio Rantanen, M.D. from Finland, and his original article became the cover story in the July issue of British Journal of Dermatology.[9] In the United Kingdom, sofas sold by Argos, Land of Leather and Walmsley Furnishing containing the chemical caused over a hundred injuries.[10][12] Argos withdrew the sofas from stores and contacted buyers to collect those that had been sold — with Land of Leather withdrawing the sofas without notifying buyers and Walmsley saying they had removed the sachets from sofas they sold after the danger came to light.[12] Complaints have been made that dates on the sofas were altered and sofas containing the sachets sold.[12] Land of Leather and Walmsley are facing a ₤10 million class action suit over their reaction to the incident, joining the manufacturer in denying the sofas are connected to their customers injuries.[12][13][14] The danger came to public attention when the BBC Watchdog program alerted consumers to the sofas.[12][13]
In the European Union the use of dimethyl fumarate for consumer products has been forbidden since 1998, and since January 2009 the import of products containing dimethyl fumarate is also forbidden.[15]
The EU Commission Decision 2009/251 of 17 March 2009 requiring Member States to ensure that products containing the biocide dimethylfumarate are not placed or made available on the market has definitely forbidden any marketing of products containing dimethyl fumarate into the European Union.[16] The ban on dimethyl fumarate as laid down in Decision 2009/251 establishes a maximum concentration of dimethyl fumarate in products of 0.1 ppm. Products containing more than 0.1 ppm dimethyl fumarate shall be withdrawn from the market and recalled by consumers as of 1 May 2009.
References
- ^ a b Acros MSDS
- ^ Schmidt, Thomas; Ak, Muharrem; Mrowietz, Ulrich (January 2007). "Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-L-cysteine--preparation of S-substituted thiosuccinic acid esters". Bioorganic & Medicinal Chemistry 15 (1): 333–42. doi:10.1016/j.bmc.2006.09.053. ISSN 0968-0896. PMID 17049250. http://www.sciencedirect.com/science/article/pii/S0968089606007851.
- ^ Schmidt, Thomas J.; Aka, Muharrem; Mrowietz, Ulrich (2007). "Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine—Preparation of S-substituted thiosuccinic acid esters". Bioorganic & Medicinal Chemistry 15 (1): 333–342. doi:10.1016/j.bmc.2006.09.053. PMID 17049250.
- ^ "Fumaric acid esters from Germany may offer help in severe psoriasis.(Dermatology)". Internal Medicine News. 1 November 2005. http://www.accessmylibrary.com/coms2/summary_0286-12505626_ITM.
- ^ "Food extract boosts cancer drug". BBC News. 8 October 2004. http://news.bbc.co.uk/nolpda/ukfs_news/hi/newsid_3727000/3727652.stm. Retrieved 1 May 2010.
- ^ http://www.msrc.co.uk/index.cfm/fuseaction/show/pageid/1679
- ^ http://www.medscape.com/viewarticle/740695
- ^ http://www.genengnews.com/gen-news-highlights/biogen-idec-reports-positive-data-from-second-phase-iii-trial-with-multiple-sclerosis-drug/81245871/
- ^ a b Rantanen, Tapio (2008). "The cause of the Chinese sofa/chair dermatitis epidemic is likely to be contact allergy to dimethylfumarate, a novel potent contact sensitizer". British Journal of Dermatology 159 (1): 218–221. doi:10.1111/j.1365-2133.2008.08622.x. PMID 18503603.
- ^ a b c "Myrkkytuoli-ihottumien syy selvisi" (in Finnish). YLE Uutiset (YLE). 2008-04-24. http://www.yle.fi/uutiset/24h/id88989.html. Retrieved 2008-06-10.
- ^ http://www.thisisnottingham.co.uk/displayNode.jsp?nodeId=176452&command=displayContent&sourceNode=134483&contentPK=20867636&folderPk=78482&pNodeId=134462
- ^ a b c d e f Bracchi, Paul (21 June 2008). "This baby was burned red raw by a sofa giving off toxic fumes. As our investigation reveals, there are hundreds of other victims". Daily Mail (London). http://www.dailymail.co.uk/femail/article-1028097/This-baby-burned-red-raw-sofa-giving-toxic-fumes-As-investigation-reveals-hundreds-victims.html.
- ^ a b BBC - Consumer - TV and radio - itchy sofas
- ^ BBC: Judge rejects 'toxic sofa' claims in burns injury cases, 18 March 2010
- ^ "Consumers: EU to ban dimethylfumarate (DMF) in consumer products, such as sofas and shoes". European Union. 2009-01-29. http://europa.eu/rapid/pressReleasesAction.do?reference=IP/09/190&format=PDF&aged=0&language=EN.
- ^ "2009/251/EC: Commission Decision of 17 March 2009". http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:074:0032:0034:EN:PDF.
External links
- Dimethyl fumarate Material Safety Data Sheet (MSDS)
Categories:- Methyl esters
- Fumarates
Wikimedia Foundation. 2010.
