- Dimethyl maleate
-
Dimethyl maleate 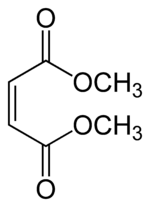 Dimethyl (Z)-but-2-enedioate
Dimethyl (Z)-but-2-enedioateIdentifiers CAS number 624-48-6 
ChemSpider 4436352 
EC number 210-848-5 ChEBI CHEBI:35460 
Jmol-3D images Image 1 - O=C(OC)\C=C/C(=O)OC
Properties Molecular formula C6H8O4 Molar mass 144.13 g/mol Appearance clear, colorless, oily liquid Density 1.15 g/cm³ Melting point -17 °C
Boiling point 204 - 207 °C
Solubility in water slightly soluble Hazards EU classification Harmful (H) NFPA 704 Flash point 95 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethyl maleate is an organic compound with the formula C6H8O4. It is the (Z)-isomer of the dimethyl ester of fumaric acid.
Contents
Synthesis
Dimethyl maleate can be synthesized from maleic anhydride and methanol, with sulfuric acid acting as acid catalyst, via a nucleophilic acyl substitution for the monomethyl ester, followed by a Fischer esterification reaction for the dimethyl ester.
Applications
Dimethyl maleate is used in many organic syntheses as a dienophile for diene synthesis. It is used as an additive and intermediate for plastics, pigments, pharmaceuticals, and agricultural products. It is also an intermediate for the production of paints, adhesives, and copolymers.[1]
Dimethyl maleate has also found use in applications where improvements in the hardness and toughness of polymer films are desired. This includes, in particular, the improvement of anti-blocking properties of copolymers of vinyl acetate with DMM. It is also used as an internal modifier to increase the glass transition temperature of styrene or vinyl chloride polymers.[2]
Chemistry
Hydrolysis of dimethyl maleate gives maleic acid, or possibly the maleic acid monomethyl ester. Hydration of the same compound gives malic acid.
See also
References
- ^ "Dimethyl maleate", Chemical Land 21
- ^ "Dimethyl maleate", Bimax Chemicals Ltd.
Categories:- Methyl esters
- Carboxylate esters
- Alkenes
Wikimedia Foundation. 2010.

