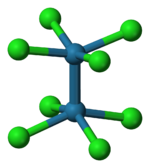
- Potassium octachlorodimolybdate
-
Potassium octachlorodimolybdate Properties Molecular formula Cl8K4Mo2 Molar mass 631.9 g/mol Appearance red crystals  octachlorodimolybdate (verify) (what is:
octachlorodimolybdate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Potassium octachlorodimolybdate is the inorganic compound with the formula K4Mo2Cl8. This red-coloured salt consists of potassium cations and the octachlorodimolybdate anion, Mo2Cl84−. The anion is of historic interest because it was one of the earliest illustrations of a quadruple bonding. The salt is usually obtained as the dihydrate.
The compound is prepared in two steps from molybdenum hexacarbonyl:[1][2]
- 2 Mo(CO)6 + 4 HO2CCH3 → Mo2(O2CCH3)4 + 2 H2 + 12 CO
- Mo2(O2CCH3)4 + 4 HCl + 4 KCl → K4Mo2Cl8 + 4 HO2CCH3
The reaction of the acetate with HCl was first described as providing trimolybdenum compounds,[3] but subsequent crystallographic analysis confirmed that the product contains the Mo2Cl84− ion with D4h symmetry. The Mo---Mo distance is 2.14 Å.[4]
References
- ^ A. B. Brignole, F. A. Cotton, Z. Dori (1972). Rhenium and Molybdenum Compounds Containing Quadruple Bonds. "Inorganic Syntheses". Inorg. Synth.. Inorganic Syntheses 13: 81–89. doi:10.1002/9780470132449.ch15. ISBN 9780470132449.
- ^ Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 0935702482.
- ^ G.B. Allison, I.R. Anderson, and J.C. Sheldon (1967). "The Preparation of Halogenotrimolybdate(II) Compounds". Australian Journal of Chemistry 20 (5): 869–876. doi:10.1071/CH9670869.
- ^ Jurij V. Brencic and F. Albert Cotton (1969). ""Octachlorodimolybdate(II) Ion. Species with a Quadruple Metal-Metal Bond". Inorg. Chem. 8: 7–10. doi:10.1021/ic50071a002.
Potassium compounds KBr · KBrO3 · KCN · KCNO · KCl · KClO3 · KClO4 · KF · KH · KHCO2 · KHCO3 · KHF2 · KHSO3 · KHSO4 · KH2AsO4 · KI · KIO3 · KIO4 · KMnO4 · KN3 · KNO2 · KNO3 · KOCN · KOH · KO2 · KPF6 · KSCN · K2CO3 · K2CrO4 · K2Cr2O7 · K2FeO4 · K2MnO4 · K2O · K2O2 · K2PtCl4 · K2PtCl6 · K2S · K2SO3 · K2SO4 · K2SO5 · K2S2O5 · K2S2O7 · K2S2O8 · K2SiO3 · K3[Fe(CN)6] · K3[Fe(C2O4)3] · K4[Fe(CN)6] · K3PO4 · Categories:
- Molybdates
- Chlorides
- Potassium compounds
Wikimedia Foundation. 2010.