- Mercury sulfide
-
Mercury sulfide 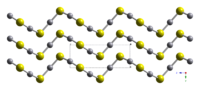
 Mercury sulfideOther names
Mercury sulfideOther namesIdentifiers CAS number 1344-48-5 
Properties Molecular formula HgS Molar mass 232.66 g/mol Density 8.10 g/cm3 Melting point 580 °C decomp.
Solubility in water insoluble Band gap 2.1 eV (direct, α-HgS) [1] Refractive index (nD) w=2.905, e=3.256, bire=0.3510 (α-HgS) [2] Hazards MSDS ICSC 0981 EU Index 080-002-00-6 EU classification Very toxic (T+)
Dangerous for the environment (N)R-phrases R26/27/28, R33, R50/53 S-phrases (S1/2), S13, S28, S45, S60, S61 Flash point Non-flammable Related compounds Other anions Mercury oxide
Mercury selenide
Mercury tellurideOther cations Zinc sulfide
Cadmium sulfide sulfide (verify) (what is:
sulfide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mercury sulfide, mercuric sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.[3]
Contents
Crystal structure
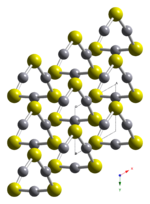
HgS is dimorphic with two crystal forms:
- red cinnabar (α-HgS, hexagonal, hP6, P3221), is the form in which mercury is most commonly found in nature.
- black, metacinnabar (β-HgS), is less common in nature and adopts the zinc blende (T2d-F-43m) crystal structure.
Crystals of red, α-HgS, are optically active. This is caused by the Hg-S helices in the structure.[4]
Preparation and chemistry
β-HgS is precipitated as a black powder when H2S is bubbled through solutions of Hg(II) salts.[5] β-HgS is unreactive to all but concentrated acids.[3]
Mercury metal is produced from the cinnabar ore by roasting in air and condensing the vapour.[3]Uses
α-HgS is used as a red pigment when it is known as vermilion. Vermilion is known to darken and this has been ascribed to conversion from red α-HgS to black β-HgS. Investigations at Pompeii where red walls when originally excavated have darkened has been ascribed to the formation of Hg-Cl compounds (e.g., corderoite, calomel, and terlinguaite) and calcium sulfate, gypsum, rather than β-HgS, which was not detected.[6]
See also
References
- ^ L. I. Berger, Semiconductor Materials (1997) CRC Press ISBN 0849389127
- ^ Webminerals
- ^ a b c Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. p. 1406. ISBN 0-08-022057-6.
- ^ Glazer, A. M.; Stadnicka K. (1986). "On the origin of optical activity in crystal structures". J. Appl. Cryst. 19 (2): 108–122. doi:10.1107/S0021889886089823.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ^ Cotte, M; Susini J, Metrich N, Moscato A, Gratziu C, Bertagnini A, Pagano M (2006). "Blackening of Pompeian Cinnabar Paintings: X-ray Microspectroscopy Analysis". Anal. Chem. 78 (21): 7484–7492. doi:10.1021/ac0612224. PMID 17073416.
External links
Mercury compounds Categories:- Sulfides
- Mercury compounds
Wikimedia Foundation. 2010.

