- Mercury(I) nitrate
-
Mercury(I) nitrate[1][2] 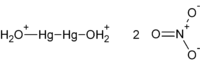 Mercury(I) nitrateOther namesMercurous nitrate
Mercury(I) nitrateOther namesMercurous nitrateIdentifiers CAS number 10415-75-5, (anhydrous)
[7782-86-7] (dihydrate)Properties Molecular formula Hg2(NO3)2 (anhydrous)
Hg2(NO3)2·2H2O (dihydrate)Molar mass 525.19 g/mol (anhydrous)
561.22 g/mol (dihydrate)Appearance white monoclinic crystals (anhydrous)
colorless crystals (dihydrate)Density ? g/cm3 (anhydrous)
4.8 g/cm3 (dihydrate)Melting point ? (anhydrous)
decomposes at 70°C (dihydrate)Solubility in water slightly soluble, reacts Hazards NFPA 704 Related compounds Other anions Mercury(I) fluoride
Mercury(I) chloride
Mercury(I) bromide
Mercury(I) iodideOther cations Mercury(II) nitrate  nitrate (verify) (what is:
nitrate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mercury(I) nitrate is a chemical compound with the formula Hg2(NO3)2. It is used in the preparation of other mercury(I) compounds, and, like all other mercury compounds, it is toxic.
Reactions
Mercury(I) nitrate is formed when elemental mercury is combined with dilute nitric acid (concentrated nitric acid will yield mercury(II) nitrate). Mercury(I) nitrate is a reducing agent which is oxidized upon contact with air.
Solutions of mercury(I) nitrate are acidic due to slow reaction with water:
- Hg2(NO3)2 + H2O → Hg2(NO3)(OH) + HNO3
Hg2(NO3)(OH) forms a yellow precipitate.
If the solution is boiled or exposed to light, mercury(I) nitrate undergoes a disproportionation reaction yielding elemental mercury and mercury(II) nitrate[2]:
- 2Hg2(NO3)2 → Hg + Hg(NO3)2
References
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–45, ISBN 0849305942
- ^ a b Patnaik, Pradyot (2003), Handbook of Inorganic Chemical Compounds, McGraw-Hill Professional, pp. 573, ISBN 0070494398, http://books.google.com/?id=Xqj-TTzkvTEC&pg=PA552&dq=%22Manganese%28II%29+acetate%22, retrieved 2009-07-20
Mercury compounds Categories:- Mercury compounds
- Nitrates
- Inorganic compound stubs
Wikimedia Foundation. 2010.

