- Chloroauric acid
-
Chloroauric acid 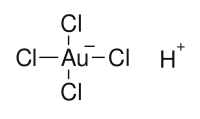
 Other namesHydrogen tetrachloroaurate,
Other namesHydrogen tetrachloroaurate,
Aurochloric acid,
Aurate(1-), tetrachloro-, hydrogen, (SP-4-1)-Identifiers CAS number 16903-35-8 
ChemSpider 26171 
Jmol-3D images Image 1 - [H+].Cl[Au-](Cl)(Cl)Cl
Properties Molecular formula HAuCl4 Molar mass 339.785 g/mol (anhydrous)
393.833 g/mol (trihydrate)
411.85 g/mol (tetrahydrate)Appearance golden yellow crystals
hygroscopicDensity 3.9 g/cm3 (tetrahydrate) Melting point 254°C
Solubility in water soluble Solubility soluble in alcohol, ether Related compounds Other anions Tetrabromoauric acid  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chloroauric acid is a inorganic compound with the formula HAuCl4. This pale yellow compound is a common precursor to gold (Au) in a variety of purposes. The term chloroauric acid is also sometimes used to describe other gold chlorides.
Contents
Preparation and structure
Chloroauric acid is obtained by dissolving gold in aqua regia followed by evaporation of these solutions.[1] Upon heating, chloroauric acid liberates hydrogen chloride, giving gold(III) chloride. This reaction is reversible: dissolving gold(III) chloride in hydrochloric acid:
In aqueous solution, chloroauric acid consists of the square planar [AuCl4]− ion. It is a common precursor to other gold coordination complexes.[2]
Reactions
Chloroauric acid is readily reduced by most metals to precipitate elemental gold.
This compound is reduced by dimethyl sulfide to give chloro(dimethyl sulfide)gold(I), which is also useful as a precursor to other gold complexes.[3]
Applications
Chloroauric acid is used as the electrolyte in the Wohlwill process for refining gold.
Generally, colloidal gold and gold nanoparticles are produced in a solution ("liquid chemical methods") by reduction of chloroauric acid with trisodium citrate, although alternative methods exist, such as the Norrish reaction and the aldehyde reduction used in Angel gilding.
References
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1057.
- ^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Marie-Claude Brandys , Michael C. Jennings and Richard J. Puddephatt (2000). "Luminescent gold(I) macrocycles with diphosphine and 4,4-bipyridyl ligands". J. Chem. Soc., Dalton Trans. (24): 4601–4606. doi:10.1039/b005251p.
Gold compounds Categories:- Gold compounds
- Acids
Wikimedia Foundation. 2010.
