- Monopotassium phosphate
-
Monopotassium phosphate 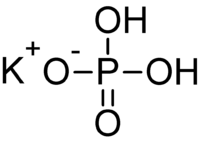 Potassium dihydrogen phosphateOther namesPotassium phosphate monobasic
Potassium dihydrogen phosphateOther namesPotassium phosphate monobasic
Phosphoric acid, monopotassium saltIdentifiers CAS number 7778-77-0 
PubChem 516951 ChemSpider 22914 
UNII 4J9FJ0HL51 
RTECS number TC6615500 Jmol-3D images Image 1 - [K+].[K+].[K+].[O-]P([O-])([O-])=O
Properties Molecular formula KH2PO4 Molar mass 136.086 g/mol Appearance White powder
deliquescentDensity 2.338 g/cm3 Melting point 252.6 °C
Boiling point 400 °C, dec
Solubility in water 22 g/100 mL (25°C) Solubility insoluble in alcohol Acidity (pKa) 7.2 Basicity (pKb) 11.9 Structure Crystal structure tetragonal Hazards MSDS External MSDS EU Index Not listed NFPA 704 Flash point Non-flammable Related compounds Other cations Monosodium phosphate
Monoammonium phosphateRelated compounds Dipotassium phosphate
Tripotassium phosphate phosphate (verify) (what is:
phosphate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Monopotassium phosphate (also potassium dihydrogen phosphate, KDP, or monobasic potassium phosphate, MKP) -- KH2PO4 -- is a soluble salt which is used as a fertilizer, a food additive and a fungicide. It is a source of phosphorus and potassium. It is also a buffering agent. When used in fertilizer mixtures with urea and ammonium phosphates, it minimizes escape of ammonia by keeping the pH at a relatively low level.
Fertilizer grade MKP contains the equivalent of 52% P2O5 and 34% K2O, and is labeled 0-52-34. MKP is often used as a nutrient source in the greenhouse trade and in hydroponics.
It is one of the components of Gatorade (used as both an emulsifier and pH buffer) and is used as an additive in cigarettes[citation needed].
At 400°C it decomposes, by loss of water, to potassium metaphosphate (KPO3)
Contents
Nonlinear optics use
As a crystal, it is noted for its non-linear optical properties. Used in optical modulators and for non-linear optics such as SHG (second-harmonic generation).
Also to be noted is KD*P, Potassium dideuterium phosphate, with slightly different properties. Highly deuterated KDP is used in nonlinear frequency conversion of laser light instead of protonated (regular) KDP due to the fact that the replacement of protons with deuterons in the crystal shifts the third overtone of the strong OH molecular stretch to longer wavelengths, moving it mostly out of the range of the fundamental line at ~1,064 nm of neodymium-based lasers. Regular KDP has absorbances at this wavelength of around 4.7-6.3%/cm of thickness while highly deuterated KDP has absorbances of typically less than .8%/cm.
Gallery
References
External links
Categories:- Second-harmonic generation
- Phosphates
- Potassium compounds
- Nonlinear optical materials
- Transparent materials
Wikimedia Foundation. 2010.



