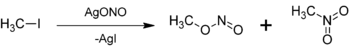- Methyl nitrite
-
Methyl nitrite 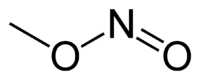
Identifiers CAS number 624-91-9 PubChem 12231 ChemSpider 11730 
Jmol-3D images Image 1 - O=NOC
Properties Molecular formula CH3NO2 Molar mass 61.04 g mol−1 Melting point -16 °C, 257 K, 3 °F
Boiling point -12 °C, 261 K, 10 °F
Hazards MSDS External MSDS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references In organic chemistry, methyl nitrite is the simplest alkyl nitrite.
Contents
Structure
At room temperature, methyl nitrite exists as a mixture of cis and trans conformers. The cis conformer is 3.13 kJ mol−1 more stable than the trans form, with an energy barrier to rotation of 45.3 kJ mol−1.[1]
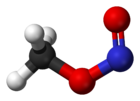
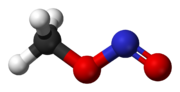
cis-methyl nitrite trans-methyl nitrite Synthesis
Methyl nitrite can be prepared by the reaction of silver nitrite with a iodomethane: Silver nitrite (AgNO2) exists in solution as the silver ion, Ag+ and the nitrite ion, NO2−. One of the lone pairs on an oxygen from nitrite ion attacks the methyl group (—CH3), releasing the iodide ion into solution.[2] Unlike silver nitrite, silver iodide is highly insoluble in water and thus forms a solid.[3] Note that nitrogen is a better nucleophile than oxygen and most nitrites would react via an SN2-like mechanism and the major product would be nitromethane. For example, sodium and potassium nitrite reacting with iodomethane would produce mostly nitromethane, with methyl nitrite as the minor product. However, the presence of the silver ion in solution has a stabilizing effect on the formation of carbocation intermediates, increasing the percent yield of methyl nitrite. In either case, some nitromethane and methyl nitrite are both formed.[2]
This compound is produced by the combustion of unleaded petrol, and might be a cause of the decline of insects, and hence that of the House Sparrow and other songbirds in Europe.[4]
See also
References
- ^ B.J. Van der Veken, R. Maas, G.A. Guirgis, H.D. Stidham, T.G. Sheehan, J.R. Durig (1990). "Infrared spectrum, ab initio calculations, barriers to internal rotation and structural parameters for methyl nitrite". Journal of Physical Chemistry 94 (10): 4029–39. doi:10.1021/j100373a028.
- ^ a b Donald L. Pavia, Gary M. Lampman, George S. Kriz (2004). Organic Chemistry. 2. Mason, Ohio: Thompson Custom Publishing. ISBN 0030148138. OCLC 236055357.
- ^ Darrell D. Ebbing, Steven D. Gammon (2005). General Chemistry (8th ed.). Boston: Houghton Mifflin. ISBN 9780618399413.
- ^ Summers-Smith, J. Denis (September 2007). "Is unleaded petrol a factor in urban House Sparrow decline?". British Birds 100: 558. ISSN 0007-0335.
External links
Alkyl nitrites ("Poppers") Amyl nitrite (isoamyl nitrite, isopentyl nitrite) · Butyl nitrite · Cyclohexyl nitrite · Ethyl nitrite · Hexyl nitrite · Isobutyl nitrite (2-methylpropyl nitrite) · Isopropyl nitrite · Methyl nitrite Categories:
Categories:- Antianginals
- Antidotes
- Methyl esters
- Alkyl nitrites
Wikimedia Foundation. 2010.