- Oxyphenisatine
-
Oxyphenisatine 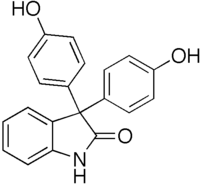 3,3-Bis(4-hydroxyphenyl)-1,3-dihydro-2H-indol-2-one[citation needed]
3,3-Bis(4-hydroxyphenyl)-1,3-dihydro-2H-indol-2-one[citation needed]Identifiers CAS number 125-13-3 
PubChem 31315 ChemSpider 29053 
UNII 3BT0VQG2GQ 
EC number 204-728-1 DrugBank DB04823 KEGG D08326 
ChEMBL CHEMBL245807 
ATC code A06 Jmol-3D images Image 1
Image 2- Oc1ccc(cc1)C1(C(=O)Nc2ccccc12)c1ccc(O)cc1
OC1=CC=C(C=C1)C1(C(=O)NC2=C1C=CC=C2)C1=CC=C(O)C=C1
Properties Molecular formula C20NH15O3 Exact mass 317.105193351 g mol-1 log P 1.398 Acidity (pKa) 9.423 Basicity (pKb) 4.574 Pharmacology Routes of
administrationOral, rectal Legal status Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Oxyphenisatine (or oxyphenisatin) is a laxative.[3] It is closely related to bisacodyl, sodium picosulfate, and phenolphthalein. Long term use is associated with liver damage,[4] and as a result, it was withdrawn in most countries in the early 1970s. The acetate derivative oxyphenisatine acetate was also once used as a laxative.
Natural chemical compounds similar to oxyphenisatine may be present in prunes,[5] but a recent review of the relevant scientific literature suggests that the laxative effect of prunes is due to other constituents including phenolic compounds (mainly neochlorogenic acids and chlorogenic acids) and sorbitol.[6]
References
- ^ a b c SciFinder Scholar, version 2004.2; Chemical Abstracts Service, Registry Number 125-13-3, accessed September 1, 2011
- ^ 21 C.F.R. 216.24
- ^ Farack, U. M.; Nell, G. (1984). "Mechanism of action of diphenolic laxatives: the role of adenylate cyclase and mucosal permeability". Digestion 30 (3): 191–194. doi:10.1159/000199105. PMID 6548720.
- ^ Kotha, P; Rake, MO; Willatt, D (1980). "Liver damage induced by oxyphenisatin". British medical journal 281 (6254): 1530. doi:10.1136/bmj.281.6254.1530. PMC 1714947. PMID 6893676. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1714947.
- ^ Baum, H. M.; Sanders, R. G.; Straub, G. J. (1951). "The occurrence of a diphenyl isatin in California prunes". Journal of the American Pharmaceutical Association 40 (7): 348–349. doi:10.1002/jps.3030400713. PMID 14850362.
- ^ Stacewicz-Sapuntzakis, M; Bowen, PE; Hussain, EA; Damayanti-Wood, BI; Farnsworth, NR (2001). "Chemical composition and potential health effects of prunes: a functional food?". Critical reviews in food science and nutrition 41 (4): 251–86. doi:10.1080/20014091091814. PMID 11401245.
Laxatives and cathartics (A06) Softeners, emollients Contact laxatives Oxyphenisatine • Bisacodyl • Dantron • Phenolphthalein • Castor oil • Senna glycosides • Cascara • Sodium picosulfate • BisoxatinBulk producers Osmotically acting laxatives Magnesium carbonate • Magnesium hydroxide • Magnesium oxide • Magnesium peroxide • Magnesium sulfate • Lactulose • Lactitol • Sodium sulfate • Pentaerythritol • Macrogol • Mannitol • Sodium phosphate • Sorbitol • Magnesium citrate • Sodium tartrate • Laminarid • Polyethylene glycol;Enemas Peripheral opioid antagonists Alvimopan • Methylnaltrexone • Oxycodone/naloxoneProstaglandins 
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it. - Oc1ccc(cc1)C1(C(=O)Nc2ccccc12)c1ccc(O)cc1
