- Sodium tartrate
-
Sodium L-tartrate[1] 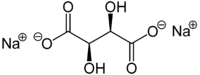 disodium (2R,3R)-2,3-dihydroxybutanedioateOther namesSal tartar; Disodium tartrate; Bisodium tartrate; Sodium L-(+)-tartrate; E335
disodium (2R,3R)-2,3-dihydroxybutanedioateOther namesSal tartar; Disodium tartrate; Bisodium tartrate; Sodium L-(+)-tartrate; E335Identifiers CAS number 868-18-8  , (anhydrous)
, (anhydrous)
[6106-24-7] (dihydrate)PubChem 13355 ChemSpider 12786 
UNII QTO9JB4MDD 
Jmol-3D images Image 1 - [Na+].[Na+].O=C([O-])C(O)C(O)C([O-])=O
Properties Molecular formula C4H4Na2O6 (anhydrous)
C4H8Na2O8 (dihydrate)Molar mass 194.051 g/mol (anhydrous)
230.082 g/mol (dihydrate)Appearance white crystals Density 1.545 g/cm3 (dihydrate) Solubility in water soluble Solubility insoluble in ethanol  tartrate (verify) (what is:
tartrate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium tartrate (Na2C4H4O6) is used as an emulsifier and a binding agent in food products such as jellies, margarine, and sausage casings. As a food additive, it is known by the E number E335.
Because its crystal structure captures a very precise amount of water, it is also a common primary standard for Karl Fischer titration, a common technique to assay water content.
References
See also
External links

This article about an organic compound is a stub. You can help Wikipedia by expanding it.
