- Nootkatone
-
Nootkatone 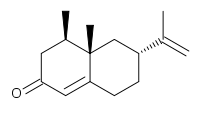 4-α,5-Dimethyl-1,2,3,4,4α,5,6,7-octahydro-7-keto-3-isopropenylnaphthaleneOther names(+)-nootkatone
4-α,5-Dimethyl-1,2,3,4,4α,5,6,7-octahydro-7-keto-3-isopropenylnaphthaleneOther names(+)-nootkatoneIdentifiers CAS number 4674-50-4 PubChem 1268142 ChemSpider 1064812 
KEGG C17914 
ChEMBL CHEMBL446299 
Jmol-3D images Image 1 - O=C2\C=C1\CC[C@@H](C(=C)C)C[C@@]1(C)[C@H](C)C2
Properties Molecular formula C15H22O Molar mass 218.33 g mol−1 Appearance Viscous yellow in its liquid form Melting point 36 °C, 309 K, 97 °F
Boiling point 170 °C, 443 K, 338 °F
Hazards S-phrases S23 S24 S25 Flash point ~ 100 °C (212 °F)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nootkatone is a natural organic compound and is the most important and expensive aromatic of grapefruit.[1] It is a sesquiterpene and a ketone.
Nootkatone was previously thought to be one of the main chemical components of the smell and flavour of grapefruits. In its solid form it is usually found as crystals. As a liquid, it is viscous and yellow. Nootkatone is typically extracted from grapefruit, but can also be manufactured with genetically modified organisms, or through the chemical or biochemical oxidation of valencene. It is also found in Alaska yellow cedar trees[2] and vetiver grass.[3]
Contents
Uses
Nootkatone in spray form has been shown as an effective repellent/insecticide against deer ticks[3][4] and lone star ticks.[4] It is also an effective repellent/insecticide against mosquitos, and may repel bed bugs, head lice and other insects.[5] It is environmentally friendly insecticide, because it is a volatile essential oil that does not persist in the environment.[5] It is nontoxic to humans, is an approved food additive,[5] and "is commonly used in foods, cosmetics, and pharmaceuticals".[3]
See also
References
- ^ Furusawa, Mai; Toshihiro Hashimoto, Yoshiaki Noma, and Yoshinori Asakawa (November 2005). "Highly Efficient Production of Nootkatone, the Grapefruit Aroma from Valencene, by Biotransformation". Chem. Pharm. Bull. 53 (11): 1513–1514. doi:10.1248/cpb.53.1513. PMID 16272746.
- ^ Panella, NA.; Dolan, MC.; Karchesy, JJ.; Xiong, Y.; Peralta-Cruz, J.; Khasawneh, M.; Montenieri, JA.; Maupin, GO. (May 2005). "Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar.". J Med Entomol 42 (3): 352–8. doi:10.1603/0022-2585(2005)042[0352:UONCFP]2.0.CO;2. PMID 15962787.
- ^ a b c Jan Suszkiw (January 2011). "Lignin + Nootkatone = Dead Ticks". USDA. http://www.ars.usda.gov/is/AR/archive/jan11/ticks0111.htm.
- ^ a b Dolan, MC.; Jordan, RA.; Schulze, TL.; Schulze, CJ.; Manning, MC.; Ruffolo, D.; Schmidt, JP.; Piesman, J. et al. (Dec 2009). "Ability of two natural products, nootkatone and carvacrol, to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey". J Econ Entomol 102 (6): 2316–24. doi:10.1603/029.102.0638. PMID 20069863.
- ^ a b c Richard Knox (April 18, 2011). "Repelling Bugs With The Essence Of Grapefruit". NPR. http://www.npr.org/2011/04/18/135468567/repelling-bugs-with-the-essence-of-grapefruit.
External links
Categories:- Flavors
- Ketones
- Sesquiterpenes
- Decalins
Wikimedia Foundation. 2010.
