- Dicyanoacetylene
-
Dicyanoacetylene 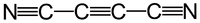

Identifiers CAS number 1071-98-3 
Properties Molecular formula C4N2 Molar mass 76.06 g/mol Density 0.907 g/cm3 Melting point 20.5 °C
Boiling point 76.5 °C
Thermochemistry Std enthalpy of
formation ΔfHo298+500.4 kJ/mol Related compounds Related compounds Carbon suboxide
Cyanogen (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula C4N2. It has a linear molecular structure, N≡C−C≡C−C≡N (often abbreviated as NC4N), with alternating triple and single covalent bonds. It can be viewed as acetylene with the two hydrogen atoms replaced by cyanide groups.
At room temperature, dicyanoacetylene is a clear liquid. Because of its high endothermic heat of formation, it can explode to carbon powder and nitrogen gas, and it burns in oxygen with a bright blue-white flame at a temperature of 5260 K (4990 °C, 9010 °F),[1] which is the hottest flame of any chemical. This high flame temperature is also the result of the absence of hydrogen, and, therefore, water as a combustion product. Because of its high specific heat, water vapor as a combustion product tends to lower the flame temperature of hydrogen containing compounds. The endothermic dissociaton of water at high temperatures above 2000 °C also prevents flame temperatures to rise above 3000 to 4000 °C.
Contents
Synthesis
Dicyanoacetylene can be prepared by passing nitrogen gas over a sample of graphite heated to temperatures between 2673 to 3000 K.[2]
As a reagent in organic chemistry
Dicyanoacetylene is a powerful dienophile because the cyanide groups are electron-withdrawing, so it is a useful reagent for Diels-Alder reactions with unreactive dienes. It even adds to the aromatic compound durene (1,2,4,5-tetramethylbenzene) to form a substituted bicyclooctatriene.[3] Only the most reactive of dienophiles can attack such aromatic compounds.
In outer space
Solid dicyanoacetylene has been detected in Titan's atmosphere by infrared spectroscopy.[4] As the seasons change on Titan, the compound condenses and evaporates in a cycle, which allows scientists on Earth to study Titanian meteorology.
As of 2006[update], the detection of dicyanoacetylene in the interstellar medium has been impossible, because its symmetry means it has no rotational microwave spectrum. However, similar asymmetric molecules like cyanoacetylene have been observed, and its presence in those environments is therefore suspected.[5]
See also
- Cyanogen, N≡C−C≡N
- Diacetylene, H−C≡C−C≡C−H
- Cyanopolyyne
References
- ^ Kirshenbaum, A. D.; and A. V. Grosse (May 1956). "The Combustion of Carbon Subnitride, NC4N, and a Chemical Method for the Production of Continuous Temperatures in the Range of 5000–6000°K". Journal of the American Chemical Society 78 (9): 2020. doi:10.1021/ja01590a075.
- ^ Ciganek, Engelbert; Krespan, Carl G. (1968-02-01). "Syntheses of dicyanoacetylene". The Journal of Organic Chemistry 33 (2): 541–544. doi:10.1021/jo01266a014.
- ^ Weis, C. D. (January 1963). "Reactions of Dicyanoacetylene". Journal of Organic Chemistry 28 (1): 74–78. doi:10.1021/jo01036a015.
- ^ Samuelson, R. E.; L. A. Mayo, M. A. Knuckles, and R. J. Khanna (August 1977). "NC4N ice in Titan's north polar stratosphere". Planetary and Space Science 45 (8): 941–948. Bibcode 1997P&SS...45..941S. doi:10.1016/S0032-0633(97)00088-3.
- ^ Kołos, Robert (August 2002). "Exotic isomers of dicyanoacetylene: A density functional theory and ab initio study". Journal of Chemical Physics 117 (5): 2063–2067. doi:10.1063/1.1489992.
Categories:- Cyanides
- Nitrides
Wikimedia Foundation. 2010.
