- Durene
-
Durene 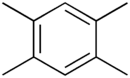
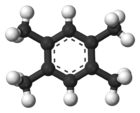 1,2,4,5-tetramethylbenzene
1,2,4,5-tetramethylbenzeneIdentifiers CAS number 95-93-2 
PubChem 7269 ChemSpider 6999 
UNII 181426CFYB 
KEGG C14534 
ChEBI CHEBI:38978 
Jmol-3D images Image 1 - c1c(c(cc(c1C)C)C)C
Properties Molecular formula C10H14 Molar mass 134.21816 Density 0.868g/cm3 Melting point 79.2 °C
Boiling point 192°C at 760mmHg
Hazards Main hazards Flammable Flash point 73.9°C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Durene, or 1,2,4,5-tetramethylbenzene, is an aromatic hydrocarbon used as a solvent. It is also an intermediate in the manufacture of pyromellitic acid, which is used for manufacturing curing agents, adhesives and coating materials. It is used in the manufacture of some raw materials for engineering plastics (polyimides) and cross-linking agent for alkyd resins.
1,2,4,5-Tetramethylbenzene has a simple proton NMR spectrum comprising two signals due to the 2 aromatic hydrogens (2H) and four methyl groups (12H). It is also readily soluble in chloroform. This compound is thus a useful internal standard.[1]
Durene is a significant byproduct that is created in the process of gasoline synthesis from methanol. This is called the "MTG (Methanol To Gasoline) process". A network consisting of a few industrial plants in New Zealand, though currently (as of March 2011) not in operation because their method of gasoline synthesis is more expensive than "traditional" methods (i.e. drilling for oil), may be reopened if gasoline prices rise above a certain level. The MTG process these plants employed produces a significant quantity of durene; their gasoline had to go through a process to reduce the level of durene to an acceptable one when constituting their final product. This was done in order to comply with New Zealand laws on gasoline composition.[2]
References
- ^ e.g. in Petr K. Sazonov, Vasyli A. Ivushkin, Galina A. Artamkina, and Irina P. Beletskaya (2003). "Metal carbonyl anions as model metal-centered nucleophiles in aromatic and vinylic substitution reactions". Arkivoc 10: 323–334. http://www.arkat-usa.org/get-file/19535/.
- ^ Packer, John; Kooy, P.; Kirk, Dr. C.M.; Wrinkles, Claire. "The Production of Methanol and Gasoline". New Zealand Institute of Chemistry. http://nzic.org.nz/ChemProcesses/energy/7D.pdf.
Categories:- Hydrocarbon solvents
- Alkylbenzenes
Wikimedia Foundation. 2010.
