- Dimethoxymethane
-
Dimethoxymethane 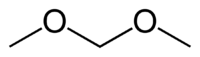
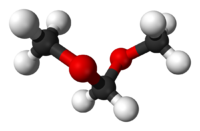 DimethoxymethaneOther namesFormal
DimethoxymethaneOther namesFormal
Formaldehyde dimethyl ether
MethylalIdentifiers CAS number 109-87-5 
PubChem 8020, 10080321 (2H3)methoxy, 18424407 sulfane, 21424378 methoxymethane ChemSpider 13837190  , 8255859 (2H3)methoxy
, 8255859 (2H3)methoxy 
EC number 203-714-2 UN number 1234 MeSH Dimethoxymethane ChEBI CHEBI:48341 ChEMBL CHEMBL15537 
RTECS number PA8750000 Beilstein Reference 1697025 Gmelin Reference 100776 Jmol-3D images Image 1 - COCOC
Properties Molecular formula C3H8O2 Molar mass 76.09 g mol−1 Exact mass 76.052429500 g mol-1 Appearance Colorless liquid Density 0.860 g cm-3 (at 20 °C) Melting point -104.8 °C, 168 K, -157 °F
Boiling point 42 °C, 315 K, 108 °F
Hazards EU classification Flammable (F)
Irritant (Xi)R-phrases R11 R36/37/38 S-phrases S9, S16, S33 Flash point -18 °C Related compounds Related Ethers Dimethoxyethane  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethoxymethane, also called methylal, is a clear colorless flammable liquid with a low boiling point, low viscosity and an excellent dissolving power. It has a chloroform-like odor and a pungent taste. It is the dimethyl acetal of formaldehyde. Dimethoxymethane is soluble in three parts water and miscible with most common organic solvents.
It can be manufactured by oxidation of methanol or by the reaction of formaldehyde with methanol. In aqueous acid, it is hydrolyzed back to formaldehyde and methanol.
It is primarily used as a solvent and in the manufacture of perfumes, resins, adhesives, paint strippers and protective coatings.
Another useful application of dimethoxymethane is to protect alcohols with a MOM ether in organic synthesis. This can be done using phosphorus pentoxide in dry dichloromethane or chloroform. This is a preferred method to using MOM-Cl. The MOM-ether can be removed using methanol and pToluene Sulphonic Acid as an alternative to aqueous acid. Due to the anomeric effect, dimethoxymethane has a preference toward the gauche conformation around the C–O bonds, instead of the anti conformation. Since it is one of the smallest molecules exhibiting this effect, which has great interest in carbohydrate chemistry, dimethoxymethane is often used for theoretical studies of the anomeric effect.
References
- Merck Index, 11th Edition, 5936
External links
Categories:- Ethers
- Ether solvents
Wikimedia Foundation. 2010.
