- Deamidation
-
Deamidation is a chemical reaction in which an amide functional group is removed from an organic compound. In biochemistry, the reaction is important in the degradation of proteins because it damages the amide-containing side chains of the amino acids asparagine and glutamine.
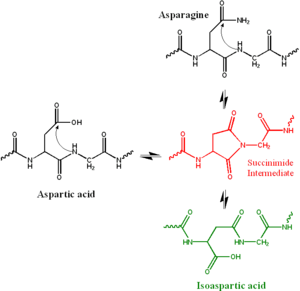
In the biochemical deamidation reaction, the side chain of an asparagine attacks the following peptide group (in black at top right of Figure), forming a symmetric succinimide intermediate (in red). The symmetry of the intermediate results in two products of its hydrolysis, either aspartate (in black at left) or in isoaspartate, which is a beta amino acid (in green at bottom right). This process is considered a deamidation because the amide in the asparagine side chain is replaced by a carboxylate group. However, a similar reaction can occur in aspartate side chains, yielding a partial conversion to isoaspartate.
Kinetics of deamidation
Deamidation reactions have been conjectured to be one of the factors that limit the useful lifetime of proteins.
Deamidation proceeds much more quickly if the susceptible amino acid is followed by a small, flexible residue such as glycine whose low steric hindrance leaves the peptide group open for attack. Deamidation reactions also proceed much more quickly at elevated pH (>10) and temperature.
References
- Clarke S. (1987) "Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins", Int. J., Peptide Protein Res., 30, 808-821. PMID 3440704
- Stephenson RC and Clarke S. (1989) "Succinimide Formation from Aspartyl and Asparaginyl Peptides as a Model for the Spontaneous Degradation of Proteins", J. Biol. Chem., 264, 6164-6170. PMID 2703484
- Robinson NE, Robinson AB. Molecular Clocks: Deamidation of Asparaginyl and Glutaminyl Residues in Peptides and Proteins. Althouse Press: Cave Junction, Ore. OCLC 56978028
See also
- Peptide bond
- Post-translational modification
Protein primary structure and posttranslational modifications General N terminus C terminus Single specific AAs Phosphorylation · Sulfation · Porphyrin ring linkage · Adenylylation · Flavin linkage · Topaquinone (TPQ) formationAspartateSuccinimide formationGlutamateDeamidation · GlycosylationTransglutaminationMethylation · Acetylation · Acylation · Adenylylation · Hydroxylation · Ubiquitination · Sumoylation · ADP-ribosylation · Deamination · Oxidative deamination to aldehyde · O-glycosylation · Imine formation · Glycation · CarbamylationDiphthamide formation · AdenylylationCrosslinks between two AAs Sulfilimine bondLysine-TyrosylquinoneLysine tyrosylquinone (LTQ) formationTryptophan-TryptophylquinoneThree consecutive AAs
(Chromophore formation)4-(p-hydroxybenzylidene)-5-imidazolinone formationCrosslinks between four AAs Secondary structure→Categories:- Substitution reactions
Wikimedia Foundation. 2010.