- Myristoylation
-
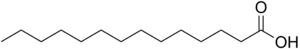 In myristoylation, a myristoyl group (derived from myristic acid, pictured above) is added.
In myristoylation, a myristoyl group (derived from myristic acid, pictured above) is added.
Myristoylation is an irreversible, co-translational (during translation) protein modification found in animals, plants, fungi, protozoans[1] and viruses. In this protein modification, a myristoyl group (derived from myristic acid) is covalently attached via an amide bond to the alpha-amino group of an N-terminal amino acid of a nascent polypeptide. It is more common on glycine residues but also occurs on other amino acids. The modification is catalyzed by the enzyme N-myristoyltransferase (NMT), and occurs most commonly on glycine residues exposed during co-translational N-terminal methionine removal. Myristoylation also occurs post-translationally, for example when previously internal glycine residues become exposed by caspase cleavage during apoptosis.
Contents
Function
Myristoylation plays a vital role in membrane targeting and signal transduction in plant responses to environmental stress.
See also
References
- Podell S and Gribskov M. (2004) "Predicting N-terminal myristoylation sites in plant proteins", BMC Genomics, 5, 37.
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ (2000) "Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis", Science 290, 1761-1765.
- ^ Kara, U. A.; Stenzel, D. J.; Ingram, L. T.; Bushell, G. R.; Lopez, J. A.; Kidson, C. (1988). "Inhibitory monoclonal antibody against a (myristylated) small-molecular-weight antigen from Plasmodium falciparum associated with the parasitophorous vacuole membrane". Infection and immunity 56 (4): 903–909. PMC 259388. PMID 3278984. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=259388.
External links
Protein primary structure and posttranslational modifications General N terminus C terminus Single specific AAs Phosphorylation · Sulfation · Porphyrin ring linkage · Adenylylation · Flavin linkage · Topaquinone (TPQ) formationAspartateGlutamateTransglutaminationMethylation · Acetylation · Acylation · Adenylylation · Hydroxylation · Ubiquitination · Sumoylation · ADP-ribosylation · Deamination · Oxidative deamination to aldehyde · O-glycosylation · Imine formation · Glycation · CarbamylationDiphthamide formation · AdenylylationCrosslinks between two AAs Sulfilimine bondLysine-TyrosylquinoneLysine tyrosylquinone (LTQ) formationTryptophan-TryptophylquinoneThree consecutive AAs
(Chromophore formation)4-(p-hydroxybenzylidene)-5-imidazolinone formationCrosslinks between four AAs Secondary structure→Categories:- Posttranslational modification
- Signal transduction
- Biochemistry stubs
Wikimedia Foundation. 2010.
