- Chlorosulfuric acid
-
Chlorosulfuric acid 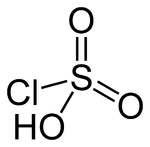
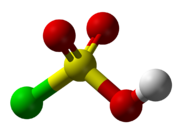 Sulfurochloridic acidOther namesChlorosulfuric acid,
Sulfurochloridic acidOther namesChlorosulfuric acid,
Chlorosulfonic acid,
Chloridosulfonic acid sulfuric chlorohydrinIdentifiers CAS number 7790-94-5 
PubChem 24638 ChemSpider 23040 
UN number 1754 RTECS number FX5730000 Jmol-3D images Image 1 - ClS(=O)(=O)O
Properties Molecular formula HSO3Cl Molar mass 116.52 g mol−1 Appearance colorless liquid that fumes in air Density 1.753 g cm−3 Melting point −80 °C
Boiling point 151–152 °C
(755 mmHg or 100.7 kPa)Solubility in water hydrolysis Solubility in other solvents reacts with alcohols
soluble in chlorocarbonsRefractive index (nD) 1.433 Structure Molecular shape tetrahedral Hazards MSDS ICSC 1039 EU Index 016-017-00-1 EU classification Corrosive (C) R-phrases R14, R35, R37 S-phrases (S2), S26, S45 NFPA 704 Related compounds Related compounds Sulfuryl chloride
Sulfuric acid acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid. It is a distillable, colorless liquid that should be handled with care. It is a hygroscopic and a powerful lachrymator.[1]
Contents
Structure and properties
Chlorosulfuric acid is a tetrahedral molecule. The formula is more descriptively written SO2(OH)Cl, but HSO3Cl is traditional. It is an intermediate, chemically and conceptually, between sulfuryl chloride (SO2Cl2) and sulfuric acid (H2SO4).[2] The compound is rarely obtained pure. Upon standing with excess sulfur trioxide, it decomposes to pyrosulfuryl chlorides:[3]
- 2 ClSO3H + SO3 → H2SO4 + S2O5Cl2
Synthesis
The industrial synthesis entails the reaction of hydrogen chloride with a solution of sulfur trioxide in sulfuric acid:[3]
-
- HCl + SO3 → ClSO3H
It can also be prepared by chlorination of sulfuric acid, written here for pedagogical purposes as SO2(OH)2, vs. the usual format H2SO4:
-
- PCl5 + SO2(OH)2 → ClSO3H + POCl3 + HCl
The latter method is more suited for laboratory-scale operations.
Applications
ClSO2OH is used to prepare sulfonic acids, which are useful in detergents and as chemical intermediates:
-
- ROH + ClSO3H → ROSO3H + HCl
An early synthesis of saccharin begins with the reaction of toluene with ClSO2OH to give the ortho- and para-toluene sulfonyl chloride derivatives:
-
- CH3C6H5 + 2ClSO2OH → CH3C6H4SO2Cl + H2SO4
Oxidation of the ortho isomer gives the benzoic acid derivative that then is cyclized with ammonia and neutralized with base to afford saccharin.
Safety
ClSO3H reacts violently with water to yield sulfuric acid and HCl. Precautions associated with HCl should be observed.
Related halosulfuric acids
- FSO2OH is a related strong acid with a diminished tendency to evolve HF.
- Bromosulfonic acid, BrSO2OH, is unstable, decomposing at its melting point of 8°C to give Br2, SO2, and H2SO4.
- Iodosulfonic acid is unknown.
References
- ^ R.J. Cremlyn, "Chlorosulfonic Acid" Springer-Verlag New York, Inc. (Royal Society of Chemistry, 2002, 300 pp. ISBN 978-0-85404-498-6
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001, pages 549-550 (discussion of XSO2OH for X = F, Cl, Br, I)
- ^ a b Joachim Maas, Fritz Baunack "Chlorosulfuric Acid" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a07_017
See also
Categories:- Oxochlorides
- Reagents for organic chemistry
- Sulfur oxoacids
- Sulfuryl compounds
- Lachrymatory agents
Wikimedia Foundation. 2010.

