- Major basic protein
-
Proteoglycan 2, bone marrow (natural killer cell activator, eosinophil granule major basic protein) Available structures PDB 1H8U, 2BRS Identifiers Symbols PRG2; BMPG; MBP; MBP1; MGC14537 External IDs OMIM: 605601 MGI: 103294 HomoloGene: 2044 GeneCards: PRG2 Gene Gene Ontology Molecular function • binding
• sugar binding
• heparin bindingCellular component • extracellular region
• transport vesicle
• cytoplasmic vesicleBiological process • immune response
• defense response to bacteriumSources: Amigo / QuickGO RNA expression pattern 
More reference expression data Orthologs Species Human Mouse Entrez 5553 19074 Ensembl ENSG00000186652 ENSMUSG00000027073 UniProt P13727 Q545D8 RefSeq (mRNA) NM_002728.4 NM_008920.4 RefSeq (protein) NP_002719.3 NP_032946.1 Location (UCSC) Chr 11:
57.15 – 57.16 MbChr 2:
84.82 – 84.82 MbPubMed search [1] [2] Proteoglycan 2, bone marrow (natural killer cell activator, eosinophil granule major basic protein), also known as PRG2, is a protein which in humans is encoded by the PRG2 gene.[1]
Contents
Function
The protein encoded by this gene is the predominant constituent of the crystalline core of the eosinophil granule. High levels of the proform of this protein are also present in placenta and pregnancy serum, where it exists as a complex with several other proteins including pregnancy-associated plasma protein A (PAPPA), angiotensinogen (AGT), and C3dg. This protein may be involved in antiparasitic defense mechanisms as a cytotoxin and helminthotoxin, and in immune hypersensitivity reactions. It is directly implicated in epithelial cell damage, exfoliation, and bronchospasm in allergic diseases.[1]
PRG2 is a 117-residue protein that predominates in eosinophil granules. It is a potent enzyme against helminths and is toxic towards bacteria and mammalian cells in vitro. The eosinophil major basic protein also causes the release of histamine from mast cells and basophils, activates neutrophils and alveolar macrophages, and is directly implicated in epithelial cell damage, exfoliation and bronchospasm in asthma.
Structure
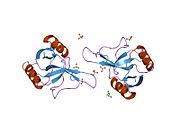
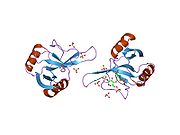
Structurally the major basic protein (MBP) is similar to lectins (sugar-binding proteins), and has a fold similar to that seen in C-type lectins. However, unlike other C-type lectins (those that bind various carbohydrates in the presence of calcium), MBP does not bind either calcium or any of the other carbohydrates that this family recognize.
Instead, MBP recognises heparan sulfate proteoglycans. Two crystallographic structures of MBP have been determined.[2][3]
Interactions
Major basic protein has been shown to interact with Pregnancy-associated plasma protein A.[4][5][6]
See also
References
- ^ a b "Entrez Gene: PRG2 proteoglycan 2, bone marrow (natural killer cell activator, eosinophil granule major basic protein)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5553.
- ^ PDB 1h8u; Swaminathan GJ, Weaver AJ, Loegering DA, Checkel JL, Leonidas DD, Gleich GJ, Acharya KR (July 2001). "Crystal structure of the eosinophil major basic protein at 1.8 A. An atypical lectin with a paradigm shift in specificity". J. Biol. Chem. 276 (28): 26197–26203. doi:10.1074/jbc.M100848200. PMID 11319227.
- ^ PDB 2brs; Swaminathan GJ, Myszka DG, Katsamba PS, Ohnuki LE, Gleich GJ, Acharya KR (November 2005). "Eosinophil-granule major basic protein, a C-type lectin, binds heparin". Biochemistry 44 (43): 14152–14158. doi:10.1021/bi051112b. PMID 16245931.
- ^ Overgaard, M T; Haaning J, Boldt H B, Olsen I M, Laursen L S, Christiansen M, Gleich G J, Sottrup-Jensen L, Conover C A, Oxvig C (Oct. 2000). "Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor". J. Biol. Chem. (UNITED STATES) 275 (40): 31128–31133. doi:10.1074/jbc.M001384200. ISSN 0021-9258. PMID 10913121.
- ^ Overgaard, Michael T; Sorensen Esben S, Stachowiak Damian, Boldt Henning B, Kristensen Lene, Sottrup-Jensen Lars, Oxvig Claus (Jan. 2003). "Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein. Disulfide structure and carbohydrate attachment". J. Biol. Chem. (United States) 278 (4): 2106–2117. doi:10.1074/jbc.M208777200. ISSN 0021-9258. PMID 12421832.
- ^ Oxvig, C; Sand O, Kristensen T, Gleich G J, Sottrup-Jensen L (Jun. 1993). "Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein". J. Biol. Chem. (UNITED STATES) 268 (17): 12243–6. ISSN 0021-9258. PMID 7685339.
External links
Further reading
- Vanhaesebroeck B, Alessi DR (2000). "The PI3K-PDK1 connection: more than just a road to PKB". Biochem. J. 346 Pt 3 (3): 561–76. doi:10.1042/0264-6021:3460561. PMC 1220886. PMID 10698680. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1220886.
- Yoshimatsu K, Ohya Y, Shikata Y et al. (1992). "Purification and cDNA cloning of a novel factor produced by a human T-cell hybridoma: sequence homology with animal lectins". Mol. Immunol. 29 (4): 537–546. doi:10.1016/0161-5890(92)90012-M. PMID 1565101.
- Barker RL, Loegering DA, Arakawa KC et al. (1990). "Cloning and sequence analysis of the human gene encoding eosinophil major basic protein". Gene 86 (2): 285–289. doi:10.1016/0378-1119(90)90292-Y. PMID 2323577.
- Gabay JE, Scott RW, Campanelli D et al. (1989). "Antibiotic proteins of human polymorphonuclear leukocytes". Proc. Natl. Acad. Sci. U.S.A. 86 (14): 5610–5614. doi:10.1073/pnas.86.14.5610. PMC 297672. PMID 2501794. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=297672.
- Wasmoen TL, McKean DJ, Benirschke K et al. (1990). "Evidence of eosinophil granule major basic protein in human placenta". J. Exp. Med. 170 (6): 2051–2063. doi:10.1084/jem.170.6.2051. PMC 2189540. PMID 2584934. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2189540.
- Barker RL, Gleich GJ, Pease LR (1988). "Acidic precursor revealed in human eosinophil granule major basic protein cDNA". J. Exp. Med. 168 (4): 1493–1498. doi:10.1084/jem.168.4.1493. PMC 2189086. PMID 3171483. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2189086.
- McGrogan M, Simonsen C, Scott R et al. (1989). "Isolation of a complementary DNA clone encoding a precursor to human eosinophil major basic protein". J. Exp. Med. 168 (6): 2295–2308. doi:10.1084/jem.168.6.2295. PMC 2189145. PMID 3199069. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2189145.
- Wasmoen TL, Bell MP, Loegering DA et al. (1988). "Biochemical and amino acid sequence analysis of human eosinophil granule major basic protein". J. Biol. Chem. 263 (25): 12559–63. PMID 3410852.
- Weller PF, Ackerman SJ, Smith JA (1988). "Eosinophil granule cationic proteins: major basic protein is distinct from the smaller subunit of eosinophil peroxidase". J. Leukoc. Biol. 43 (1): 1–4. PMID 3422083.
- Kristensen T, Oxvig C, Sand O et al. (1994). "Amino acid sequence of human pregnancy-associated plasma protein-A derived from cloned cDNA". Biochemistry 33 (6): 1592–1598. doi:10.1021/bi00172a040. PMID 7508748.
- Oxvig C, Haaning J, Højrup P, Sottrup-Jensen L (1994). "Location and nature of carbohydrate groups in proform of human major basic protein isolated from pregnancy serum". Biochem. Mol. Biol. Int. 33 (2): 329–36. PMID 7524900.
- Bonno M, Oxvig C, Kephart GM et al. (1994). "Localization of pregnancy-associated plasma protein-A and colocalization of pregnancy-associated plasma protein-A messenger ribonucleic acid and eosinophil granule major basic protein messenger ribonucleic acid in placenta". Lab. Invest. 71 (4): 560–6. PMID 7526035.
- Li MS, Sun L, Satoh T et al. (1995). "Human eosinophil major basic protein, a mediator of allergic inflammation, is expressed by alternative splicing from two promoters". Biochem. J. 305 ( Pt 3): 921–7. PMC 1136346. PMID 7531438. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1136346.
- Oxvig C, Haaning J, Kristensen L et al. (1995). "Identification of angiotensinogen and complement C3dg as novel proteins binding the proform of eosinophil major basic protein in human pregnancy serum and plasma". J. Biol. Chem. 270 (23): 13645–13651. doi:10.1074/jbc.270.23.13645. PMID 7539791.
- Oxvig C, Sand O, Kristensen T et al. (1993). "Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein". J. Biol. Chem. 268 (17): 12243–6. PMID 7685339.
- Levi-Schaffer F, Lacy P, Severs NJ et al. (1995). "Association of granulocyte-macrophage colony-stimulating factor with the crystalloid granules of human eosinophils". Blood 85 (9): 2579–86. PMID 7727786.
- Oxvig C, Gleich GJ, Sottrup-Jensen L (1994). "Localization of disulfide bridges and free sulfhydryl groups in human eosinophil granule major basic protein". FEBS Lett. 341 (2–3): 213–217. doi:10.1016/0014-5793(94)80459-1. PMID 8137941.
- Shikata Y, Hayashi Y, Yoshimatsu K et al. (1993). "Pro-major basic protein has three types of sugar chains at the pro-portion". Biochim. Biophys. Acta 1163 (3): 243–9. doi:10.1016/0167-4838(93)90158-N. PMID 8507662.
- Nittoh T, Watanabe M, Okayama H et al. (1996). "Cloning of cDNA for rat eosinophil major basic protein". Biochim. Biophys. Acta 1264 (3): 261–4. PMID 8547309.
- Delcommenne M, Tan C, Gray V et al. (1998). "Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase". Proc. Natl. Acad. Sci. U.S.A. 95 (19): 11211–11216. doi:10.1073/pnas.95.19.11211. PMC 21621. PMID 9736715. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=21621.
PDB gallery Antimicrobial peptides: Granulocyte granule contents Azurophilic granules (1°) Specific granules (2°) Cathepsin · Major basic protein · Eosinophil cationic protein · Eosinophil peroxidase · Eosinophil-derived neurotoxinsee also platelet alpha-granule, dense granule
This enzyme-related article is a stub. You can help Wikipedia by expanding it.