- Diisopropyl azodicarboxylate
-
Diisopropyl azodicarboxylate 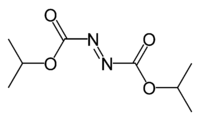
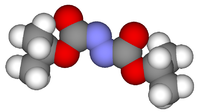 Diisopropyl azodicarboxylateOther namesDIAD
Diisopropyl azodicarboxylateOther namesDIADIdentifiers CAS number 2446-83-5 PubChem 5363146 ChemSpider 4515532 
Jmol-3D images Image 1
Image 2- O=C(OC(C)C)\N=N/C(OC(C)C)=O
O=C(/N=N/C(=O)OC(C)C)OC(C)C
Properties Molecular formula C8H14N2O4 Molar mass 202.21 g mol−1 Density 1.027 g/cm3 Melting point 3-5 °C
Boiling point 75 °C at 0.25 mmHg
Solubility in water insoluble Hazards MSDS Sigma-Aldrich EU classification Flammable (F)
Irritant (Xi)
Env. Danger (N)R-phrases R5, R11, R36, R37, R38, R43, R51, R53 S-phrases S16, S26, S29, S36, S37, S39, S47, S61 Flash point 106°C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diisopropyl azodicarboxylate (DIAD) is the diisopropyl ester of azodicarboxylic acid. It is used as a reagent in the production of many organic compounds. It is often used in the Mitsunobu reaction[1] where it serves as an oxidizer of triphenylphosphine to triphenylphosphine oxide. It has also be used to generate aza-Baylis-Hillman adducts with acrylates.[2] It can also serve as a selective deprotectant of N-benzyl groups in the presence of other protecting groups.[3]
It is sometimes preferred to diethyl azodicarboxylate (DEAD) because it is more hindered, and thus will not form hydrazide byproducts.
References
- ^ "Fluka DIAD on Sigma-Aldrich". https://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/11626. Retrieved 2008-11-18.
- ^ Shi, Min and Zhao, Gui-Ling (2004). "Aza-Baylis–Hillman reactions of diisopropyl azodicarboxylate or diethyl azodicarboxylate with acrylates and acrylonitrile". Tetrahedron 60 (9): 2083–2089. doi:10.1016/j.tet.2003.12.059.
- ^ Kroutil, J.; Trnka, T.; and Cerny, M. (2004). "Improved procedure for the selective N-debenzylation of benzylamines by diisopropyl azodicarboxylate". Synthesis 3 (3): 446–450. doi:10.1055/s-2004-815937.

This article about an organic compound is a stub. You can help Wikipedia by expanding it. - O=C(OC(C)C)\N=N/C(OC(C)C)=O
