- Mitsunobu reaction
-
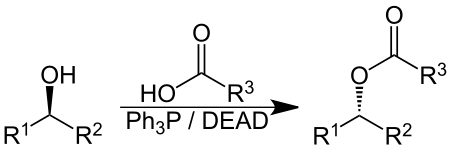
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD).[1] The alcohol undergoes an inversion of stereochemistry. It was discovered by Oyo Mitsunobu (1934–2003).
Several reviews have been published.[2] [3][4][5] [6]Contents
Reaction mechanism
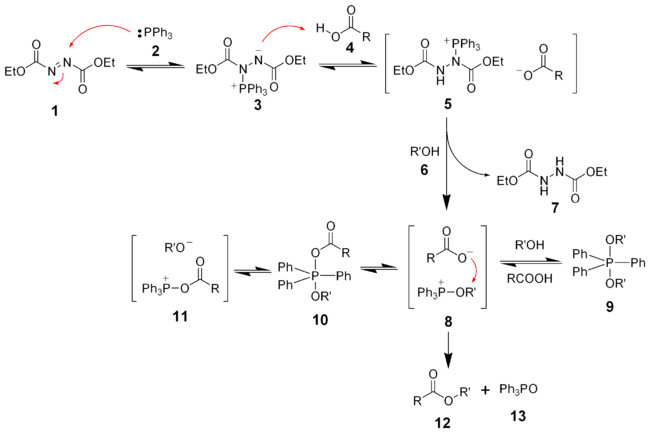
The reaction mechanism of the Mitsunobu reaction is fairly complex. The identity of intermediates and the roles they play has been the subject of debate.
Initially, the triphenyl phosphine (2) makes a nucleophilic attack upon diethyl azodicarboxylate (1) producing a betaine intermediate 3, which deprotonates the carboxylic acid (4) to form the ion pair 5. DEAD itself deprotonates the alcohol (6) forming an alkoxide that can form the key oxyphosphonium ion 8. The ratio and interconversion of intermediates 8 - 11 depend on the carboxylic acid pKa and the solvent polarity.[7][8][9] Although several phosphorus intermediates are present, the attack of the carboxylate anion upon intermediate 8 is the only productive pathway forming the desired product 12 and triphenylphosphine oxide (13).
Hughes et al. have found that the formation of the ion pair 5 is very fast. The formation of the oxyphosphonium intermediate 8 is slow and facilitated by the alkoxide. Therefore, the overall rate of reaction is controlled by carboxylate basicity and solvation.[10]
Order of addition of reagents
The order of addition of the reagents of the Mitsunobu reaction can be important. Typically, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine in tetrahydrofuran or other suitable solvent (e.g. diethyl ether), cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. If this is unsuccessful, then preforming the betaine may give better results. To preform the betaine, add DEAD to triphenylphosphine in tetrahydrofuran at 0 °C, followed by the addition of the alcohol and finally the acid.[11]
Variations
Other nucleophilic functional groups
Many other functional groups can serve as nucleophiles besides carboxylic acids. For the reaction to be successful, the nucleophile must have a pKa less than 15.
Nucleophile Product hydrazoic acid alkyl azide imide substituted imide[12] phenol alkyl aryl ether sulfonamide substituted sulfonamide[13] Modifications
Several modifications to the original reagent combination have been developed in order to simplify the separation of the product and avoid production of so much chemical waste. One variation of the Mitsunobu Reaction uses resin-bound triphenylphoshine and uses di-tert-butylazodicarboxylate instead of DEAD. The oxidized triphenylphosphine resin can be removed by filtration, and the di-tert-butylazodicarboxylate byproduct is removed by treatment with trifluoroacetic acid.[14] Bruce H. Lipshutz has developed an alternative to DEAD, Di-(4-chlorobenzyl)azodicarboxylate (DCAD) where the hydrazine by-product can be easily removed by filtration and recycled back to DCAD.[15]
A modification has also been reported in which DEAD can be used in catalytic versus stoichiometric quantites, however this procedure requires the use of stoichiometric (diacetoxyiodo)benzene to oxidise the hydrazine by-product back to DEAD.[16]
Phosphorane reagents
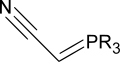
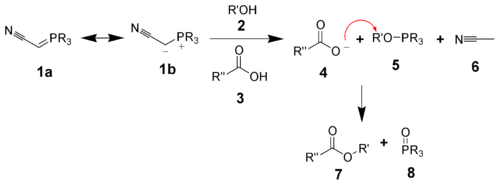
Tsunoda et al. have shown that one can combine the triphenylphosphine and the diethyl azodicarboxylate into one reagent: a phosphorane ylide. Both (cyanomethylene)trimethylphosphorane (CMMP, R = Me) and (cyanomethylene)tributylphosphorane (CMBP, R = Bu) have proven particularly effective.[17]
The ylide acts as both the reducing agent and the base. The byproducts are acetonitrile (6) and the trialkylphosphine oxide (8).
Uses
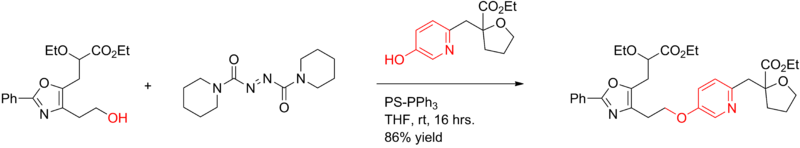
The Mitsunobu reaction has been applied in the synthesis of aryl ethers:[18]
With these particular reactants the conversion with DEAD fails because the phenol is only weakly acidic. Instead the related 1,1'-(azodicarbonyl)dipiperidine (ADDP) is used of which the betaine intermediate is a stronger base. The phosphine is a polymer-supported triphenylphosphine (PS-PPh3).
References
- ^ Mitsunobu, O.; Yamada, Y. Bull. Chem. Soc. Japan 1967, 40, 2380-2382.
- ^ The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products Mitsunobu, O. Synthesis 1981, 1-28. (Review)
- ^ Castro, B. R. Org. React. 1983, 29, 1. (Review)
- ^ Hughes, D. L. Org. React. 1992, 42, 335-656. (Review)
- ^ Hughes, D. L. Org. Prep. 1996, 28, 127-164. (Review)
- ^ Kumara Swamy, K. C.; Bhuvan Kumar, N. N.; Balaraman, E.; Pavan Kumar, K. V. P., "Mitsunobu and Related Reactions: Advances and Applications" Chem. Rev. 2009, 109, 2551-2651.
- ^ Grochowski, E. H., B. D.; Kupper, R. J.; Michejda, C. J. J. Am. Chem. Soc. 1982, 104, 6876-6877. (doi:10.1021/ja00388a110)
- ^ Camp, D. J., I. D. J. Org. Chem. 1989, 54, 3045-3049. (doi:10.1021/jo00274a016)
- ^ Camp, D. J., I. D. J. Org. Chem. 1989, 54, 3049-3054. (doi:10.1021/jo00274a017)
- ^ Hughes, D. L. R., R. A.; Bergan, J. J.; Grabowski, E. J. J. J. Am. Chem. Soc. 1988, 110, 6487-6491. (doi:10.1021/ja00227a032)
- ^ Volante, R. Tetrahedron Lett. 1981, 22, 3119.
- ^ Hegedus, L. S.; Holden, M. S.; McKearin, J. M. Organic Syntheses, Coll. Vol. 7, p.501 (1990); Vol. 62, p.48 (1984). (Article)
- ^ Kurosawa, W.; Kan, T.; Fukuyama, T. Organic Syntheses, Coll. Vol. 10, p.482 (2004); Vol. 79, p.186 (2002). (Article)
- ^ Pelletier, J. C.; Kincaid, S. (2000). "Mitsunobu reaction modifications allowing product isolation without chromatography: application to a small parallel library". Tetrahedron Lett. 41 (6): 797–800. doi:10.1016/S0040-4039(99)02214-5.
- ^ Lipshutz, B. H.; Chung, D. W.; Rich. B.; Corral, R. (2006). "Simplification of the Mitsunobu Reaction. Di-p-chlorobenzyl Azodicarboxylate: A New Azodicarboxylate". Org. Lett. 8 (22): 5069–5072. doi:10.1021/ol0618757.
- ^ Toy, Patrick; But, Tracy (2006). "Organocatalytic Mitsunobu Reactions". Journal of the American Chemical Society 128 (30): 9636. doi:10.1021/ja063141v.
- ^ Tsunoda, T.; Nagino, C.; Oguri, M.; Itô, S. Tetrahedron Lett. 1996, 37, 2459.
- ^ Humphries, P. S.; Do, Q. -Q. T.; Wilhite, D. M. (2006). "ADDP and PS-PPh3: an efficient Mitsunobu protocol for the preparation of pyridine ether PPAR agonists". Beilstein journal of organic chemistry 2: 21. doi:10.1186/1860-5397-2-21. PMC 1705810. PMID 17076898. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1705810.
External links
- The Mitsunobu Reaction by Kevin Jantzi
See also
Categories:- Substitution reactions
- Name reactions
Wikimedia Foundation. 2010.