- Death effector domain
-
Death effector domain 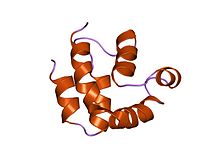
structure of the FADD (Mort1) death-effector domain.[1] Identifiers Symbol DED Pfam PF01335 InterPro IPR001875 SMART DED PROSITE PS50168 SCOP 1a1z CDD cd00045 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary The death-effector domain (DED) is a protein interaction domain found to regulate a variety of cellular signalling pathways.[2] The DED domain is found in inactive procaspases (cysteine proteases) and proteins that regulate caspase activation in the apoptosis cascade such as FAS-associating death domain-containing protein (FADD). FADD recruits procaspase 8 and procaspase 10 into a death induced signaling complex (DISC). This recruitment is mediated by a homotypic interaction between the procaspase DED and a second DED that is death effector domain in an adaptor protein that is directly associated with activated TNF receptors. Complex formation allows proteolytic activation of procaspase into the active caspase form which results in the initiation of apoptosis (cell death). Structurally the DED domain are a subclass of protein motif known as the death fold and contains 6 alpha helices, that closely resemble the structure of the Death domain.
References
- ^ Eberstadt M, Huang B, Chen Z, et al. (April 1998). "NMR structure and mutagenesis of the FADD (Mort1) death-effector domain". Nature 392 (6679): 941–5. doi:10.1038/31972. PMID 9582077.
- ^ Valmiki MG, Ramos JW (March 2009). "Death effector domain-containing proteins". Cell. Mol. Life Sci. 66 (5): 814–30. doi:10.1007/s00018-008-8489-0. PMID 18989622.
External links
Protein domains 
This protein-related article is a stub. You can help Wikipedia by expanding it.
