- Death domain
-
Death domain 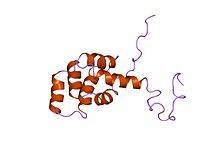
Structure of the Fas (APO-1/CD95) death domain.[1] Identifiers Symbol Death Pfam PF00531 InterPro IPR000488 SMART DEATH PROSITE PDOC50017 SCOP 1ddf CDD cd01670 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary The death domain (DD) is a protein interaction module composed of a bundle of six alpha-helices. DD is a subclass of protein motif known as the death fold and is related in sequence and structure to the death effector domain (DED) and the caspase recruitment domain (CARD), which work in similar pathways and show similar interaction properties.[2] DD bind each other forming oligomers. Mammals have numerous and diverse DD-containing proteins.[3] Within these proteins, the DD domains can be found in combination with other domains, including: CARDs, DEDs, ankyrin repeats, caspase-like folds, kinase domains, leucine zippers, leucine-rich repeats (LRR), TIR domains, and ZU5 domains.[4]
Some DD-containing proteins are involved in the regulation of apoptosis and inflammation through their activation of caspases and NF-kappaB, which typically involves interactions with TNF (tumour necrosis factor) cytokine receptors.[5][6] In humans, eight of the over 30 known TNF receptors contain DD in their cytoplasmic tails; several of these TNF receptors use caspase activation as a signaling mechanism. The DD mediates self-association of these receptors, thus giving the signal to downstream events that lead to apoptosis. Other DD-containing proteins, such as ankyrin, MyD88 and pelle, are probably not directly involved in cell death signalling. DD-containing proteins also have links to innate immunity, communicating with Toll-like receptors through bipartite adapter proteins such as MyD88.[7]
References
- ^ Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW (1996). "NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain". Nature 384 (6610): 638–41. doi:10.1038/384638a0. PMID 8967952.
- ^ Weber CH, Vincenz C (August 2001). "The death domain superfamily: a tale of two interfaces?". Trends Biochem. Sci. 26 (8): 475–81. doi:10.1016/S0968-0004(01)01905-3. PMID 11504623.
- ^ Feinstein E, Kimchi A, Wallach D, Boldin M, Varfolomeev E (September 1995). "The death domain: a module shared by proteins with diverse cellular functions". Trends Biochem. Sci. 20 (9): 342–4. doi:10.1016/S0968-0004(00)89070-2. PMID 7482697.
- ^ Reed JC, Doctor KS, Godzik A (June 2004). "The domains of apoptosis: a genomics perspective". Sci. STKE 2004 (239): re9. doi:10.1126/stke.2392004re9. PMID 15226512.
- ^ Wajant H (2003). "Death receptors". Essays Biochem. 39: 53–71. PMID 14585074.
- ^ Bhardwaj A, Aggarwal BB (September 2003). "Receptor-mediated choreography of life and death". J. Clin. Immunol. 23 (5): 317–32. doi:10.1023/A:1025319031417. PMID 14601641.
- ^ O'Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C (2003). "Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors". J. Endotoxin Res. 9 (1): 55–9. doi:10.1179/096805103125001351. PMID 12691620.
This article includes text from the public domain Pfam and InterPro IPR000488
Categories:- Protein domains
Wikimedia Foundation. 2010.
