- Galangin
-
Galangin 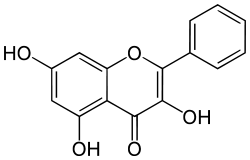 3,5,7-trihydroxy-2-phenylchromen-4-oneOther namesNorizalpinin
3,5,7-trihydroxy-2-phenylchromen-4-oneOther namesNorizalpinin
3,5,7-Trihydroxyflavone
3,5,7-triOH-FlavoneIdentifiers CAS number 548-83-4 
PubChem 5281616 ChemSpider 4444935 
UNII 142FWE6ECS 
KEGG C10044 
ChEBI CHEBI:5262 
ChEMBL CHEMBL309490 
IUPHAR ligand 410 Jmol-3D images Image 1 - O=C1c3c(O/C(=C1/O)c2ccccc2)cc(O)cc3O
Properties Molecular formula C15H10O5 Molar mass 270.24 g/mol Exact mass 270.052823 u Melting point 214-215 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Galangin is a flavonol found in high concentrations in Alpinia officinarum (lesser galangal).[1] It is also found in the galangal rhizome (Alpinia galanga)[2] and in propolis.[3] Galangin has been shown to slow the increase and growth of breast tumor cells in vitro.[4][5]
References
- ^ Ciolino, H. P.; Yeh, G. C. (1999). "The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor". British Journal of Cancer 79 (9/10): 1340–1346. doi:10.1038/sj.bjc.6690216.
- ^ Kaur, A.; Singh, R.; Dey, C. S.; Sharma, S. S.; Bhutani, K. K.; Singh, I. P. (2010). "Antileishmanial phenylpropanoids from Alpinia galanga (Linn.) Willd". Indian Journal of Experimental Biology 48 (3): 314–317. PMID 21046987. http://nopr.niscair.res.in/bitstream/123456789/7407/1/IJEB%2048(3)%20314-317.pdf.
- ^ Tosi, E; Re, E; Ortega, M; Cazzoli, A (2007). "Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli". Food Chemistry 104: 1025. doi:10.1016/j.foodchem.2007.01.011.
- ^ So, F. V.; Guthrie, N.; Chambers, A. F.; Moussa, M.; Carroll, K. K. (1996). "Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices". Nutrition and Cancer 26 (2): 167–181. doi:10.1080/01635589609514473. PMID 8875554.
- ^ So, F.; Guthrie, N.; Chambers, A. F.; Carroll, K. K. (1997). "Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen". Cancer Letters 112 (2): 127–133. doi:10.1016/S0304-3835(96)04557-0. PMID 9066718.
External links
Backbone Aglycones3-Hydroxyflavone (synthetic) and derivativesFlavonols AglyconesFisetin | Galangin | Gossypetin | Herbacetin | Kaempferol | Morin | Myricetin | Quercetagetin | QuercetinGlycosidesAstragalin | CTN-986 | Eupalin | Guaijaverin (quercetin 3-O-arabinoside) | Heliosin (Quercetin 3-digalactoside) | Hyperoside | Isoquercitin | Kaempferitrin | Myricetin 3-O-rutinoside | Myricitrin | Quercetin-3-sophorodide | Quercitrin | Rhodionin | Rhodiosin | Robinin | Rutin | SpiraeosideO-Methylated flavonols Aglycones5-O-methylmyricetin | Annulatin | Ayanin | Axillarin | Azaleatin | Brickellin | Centaureidin | Chrysosplenetin | Combretol | Ermanin | Eupatolitin | Eupalitin | Europetin | Isorhamnetin | Jaceidin | Kaempferide | Kumatakenin | Laricitrin | Natsudaidain | Ombuin | Pachypodol | Patuletin | Retusin | Mearnsetin | Rhamnazin | Rhamnetin | Santin | Spinacetin | Syringetin | TamarixetinGlycosidesAzalein | Centaurein | Eupatolin | Jacein | Patulitrin | Tamarixetin 7-rutinoside | XanthorhamninDerivative flavonols GlycosidesAmurensin | Icariin | Rutin SPyranoflavonols AglyconesKaranjachromeneFuranoflavonols AglyconesKaranjinGlycosidesPongamoside A, B and CSemisynthetic GlycosidesThis article about a natural phenol is a stub. You can help Wikipedia by expanding it.
