- Myricetin
-
Myricetin 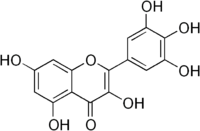 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)- 4-chromenoneOther namesCannabiscetin
3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)- 4-chromenoneOther namesCannabiscetin
Myricetol
MyricitinIdentifiers CAS number 529-44-2 
PubChem 5281672 ChemSpider 4444991 
DrugBank DB02375 ChEBI CHEBI:18152 
ChEMBL CHEMBL164 
Jmol-3D images Image 1 - Oc1cc(O)c2c(=O)c(O)c (oc2c1)c3cc(O)c(O)c(O)c3
Properties Molecular formula C15H10O8 Molar mass 318.2351 g/mol Exact mass 318.037567 u  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Myricetin is a naturally occurring flavonol, a flavonoid found in many grapes, berries, fruits, vegetables, herbs, as well as other plants. Walnuts are a rich dietary source. Trace amounts can be found as glycosides.[1] It is one of the phenolic compounds present in red wine.[2]
Myricetin has antioxidant properties. In vitro research suggests that myricetin in high concentrations can modify LDL cholesterol such that uptake by white blood cells is increased. A Finnish study correlated high myricetin consumption with lowered rates of prostate cancer.[3]
Another 8-year study found that three flavonols (kaempferol, quercetin, and myricetin) reduced the risk of pancreatic cancer by 23 percent.[4]
Contents
Metabolism
Glycosides
- Myricitrin is a rhamnoside of myricetin.
- Myricetin 3-O-rutinoside
O-méthylations
Laricitrin is formed from myricetin by the action of the enzyme myricetin O-methyltransferase.[5]
References
- ^ Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. Koo Hui Miean and Suhaila Mohamed, Faculty of Food Science and Biotechnology, University Putra Malaysia, 43400 Serdang Selangor, Malaysia
- ^ The red wine phenolics piceatannol and myricetin act as agonists for estrogen receptor in human breast cancer cells. M Maggiolini, A G Recchia, D Bonofiglio, S Catalano, A Vivacqua, A Carpino, V Rago, R Rossi and S Andò, Journal of Molecular Endocrinology (2005) 35 269-281
- ^ Knekt P, Kumpulainen J, Järvinen R, et al. (September 2002). "Flavonoid intake and risk of chronic diseases". Am. J. Clin. Nutr. 76 (3): 560–8. PMID 12198000. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=12198000.
- ^ Nöthlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN (October 2007). "Flavonols and pancreatic cancer risk: the multiethnic cohort study". Am. J. Epidemiol. 166 (8): 924–31. doi:10.1093/aje/kwm172. PMID 17690219. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=17690219.
- ^ Syringetin biosynthetis pathway on metacyc.org
Backbone Aglycones3-Hydroxyflavone (synthetic) and derivativesFlavonols AglyconesFisetin | Galangin | Gossypetin | Herbacetin | Kaempferol | Morin | Myricetin | Quercetagetin | QuercetinGlycosidesAstragalin | CTN-986 | Eupalin | Guaijaverin (quercetin 3-O-arabinoside) | Heliosin (Quercetin 3-digalactoside) | Hyperoside | Isoquercitin | Kaempferitrin | Myricetin 3-O-rutinoside | Myricitrin | Quercetin-3-sophorodide | Quercitrin | Rhodionin | Rhodiosin | Robinin | Rutin | SpiraeosideO-Methylated flavonols Aglycones5-O-methylmyricetin | Annulatin | Ayanin | Axillarin | Azaleatin | Brickellin | Centaureidin | Chrysosplenetin | Combretol | Ermanin | Eupatolitin | Eupalitin | Europetin | Isorhamnetin | Jaceidin | Kaempferide | Kumatakenin | Laricitrin | Natsudaidain | Ombuin | Pachypodol | Patuletin | Retusin | Mearnsetin | Rhamnazin | Rhamnetin | Santin | Spinacetin | Syringetin | TamarixetinGlycosidesAzalein | Centaurein | Eupatolin | Jacein | Patulitrin | Tamarixetin 7-rutinoside | XanthorhamninDerivative flavonols GlycosidesAmurensin | Icariin | Rutin SPyranoflavonols AglyconesKaranjachromeneFuranoflavonols AglyconesKaranjinGlycosidesPongamoside A, B and CSemisynthetic GlycosidesCategories:- Flavonols
- Phenolic compounds in wine
- Xanthine oxidase inhibitors
Wikimedia Foundation. 2010.
