- Bixin
-
Bixin[1] 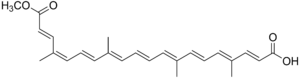
 (2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acidOther namescis-Bixin; α-Bixin; 9-cis-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester
(2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acidOther namescis-Bixin; α-Bixin; 9-cis-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl esterIdentifiers CAS number 6983-79-5,
39937-23-0 (trans-Bixin)PubChem 5281226 ChemSpider 4444638 UNII 9L7T4VB66G Jmol-3D images Image 1 - O=C(O)\C=C\C(=C\C=C\C(=C\C=C\C=C(\C=C\C=C(/C=C/C(=O)OC)C)C)C)C
- InChI=1/C25H30O4/c1-20(12-8-14-22(3)16-18-24(26)27)10-6-7-11-21(2)13-9-15-23(4)17-19-25(28)29-5/h6-19H,1-5H3,(H,26,27)/b7-6+,12-8+,13-9+,18-16+,19-17+,20-10+,21-11+,22-14+,23-15-
Key: RAFGELQLHMBRHD-SLEZCNMEBU
Properties Molecular formula C25H30O4 Molar mass 394.5 g mol−1 Appearance Orange crystals Solubility in water Insoluble Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Bixin is an apocarotenoid found in annatto, a natural food coloring obtained from the seeds of the achiote tree (Bixa orellana). Annatto seeds contain about 5% pigments, which consist of 70-80% bixin.[2]
Bixin is chemically unstable when isolated and converts via isomerization into trans-bixin (β-bixin), the double-bond isomer.[1]
Bixin is soluble in fats but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
References
- ^ a b Merck Index, 11th Edition, 1320
- ^ Executive Summary Bixin, National Toxicology Program
Carotenes (C40) α-Carotene · β-Carotene · γ-Carotene · δ-Carotene · ε-Carotene · ζ-Carotene · Lycopene · Neurosporene · Phytoene · PhytoflueneXanthophylls (C40) Antheraxanthin · Astaxanthin · Canthaxanthin · Citranaxanthin · Cryptoxanthin · Diadinoxanthin · Diatoxanthin · Dinoxanthin · Flavoxanthin · Fucoxanthin · Lutein · Neoxanthin · Rhodoxanthin · Rubixanthin · Violaxanthin · ZeaxanthinApocarotenoids (C<40) Vitamin A retinoids (C20) Retinoid drugs Acitretin · Alitretinoin · Bexarotene · Etretinate · Fenretinide · Isotretinoin · Tazarotene · TretinoinCategories:- Apocarotenoids
Wikimedia Foundation. 2010.