- Molybdenum trioxide
-
Molybdenum trioxide 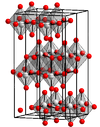
 Molybdenum trioxideOther namesMolybdic anhydride
Molybdenum trioxideOther namesMolybdic anhydride
Molybdite
Molybdic trioxideIdentifiers CAS number 1313-27-5  ?
?Properties Molecular formula MoO3 Molar mass 143.94 g mol−1 Appearance yellow or light blue solid[1] Density 4.69 g/cm3, solid Melting point 795 °C
Boiling point 1155 °C
Solubility in water 0.1066 g/100 mL (18 °C)
2.055 g/100 mL (70 °C)Structure Crystal structure orthorhombic Coordination
geometrysee text Thermochemistry Std enthalpy of
formation ΔfHo298−745.17 kJ/mol Standard molar
entropy So29877.78 J K−1 mol−1 Hazards MSDS External MSDS EU Index 042-001-00-9 EU classification Carc. Cat. 3
Harmful (Xn)
Irritant (Xi)R-phrases R36/37, R40 S-phrases (S2), S22, S36/37 Flash point Non-flammable Related compounds Other cations Chromium trioxide
Tungsten trioxideRelated molybdenum oxides Molybdenum dioxide
"Molybdenum blue"Related compounds Molybdic acid
Sodium molybdateSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Molybdenum trioxide is chemical compound with the formula MoO3. This compound is produced on the largest scale of any molybdenum compound. It occurs as the rare mineral molybdite. Its chief application is as an oxidation catalyst and as a raw material for the production of molybdenum metal.
The oxidation state of molybdenum in this compound is +6.
Contents
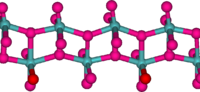
Structure
In the gas phase, three oxygen atoms are double bonded to the central molybdenum atom. In the solid state, anhydrous MoO3 is composed of layers of distorted MoO6 octahedra in an orthorhombic crystal. The octahedra share edges and form chains which are cross-linked by oxygen atoms to form layers. The octahedra have one short molydenum-oxygen bond to a non-bridging oxygen.[2]
Preparation and principal reactions
MoO3 is produced industrially by roasting molybdenum disulfide, the chief ore of molybdenum:
- 2 MoS2 + 7 O2 → 2 MoO3 + 4 SO2
The laboratory synthesis entails the acidification of aqueous solutions of sodium molybdate with perchloric acid:[3]
- Na2MoO4 + H2O + 2 HClO4 → MoO3(H2O)2 + 2 NaClO4
The dihydrate loses water readily to give the monohydrate. Both are bright yellow in color.
Molybdenum trioxide dissolves slightly in water to give "molybdic acid." In base, it dissolves to afford the molybdate anion.
Uses
Molybdenum trioxide is used to manufacture molybdenum metal, which serves as an additive to steel and corrosion-resistant alloys. The relevant conversion entails treatment of MoO3 with hydrogen at elevated temperatures:
- MoO3 + 3 H2 → Mo + 3 H2O
It is also a component of the co-catalyst used in the industrial production of acrylonitrile by the oxidation of propene and ammonia.
Because of its layered structure and the ease of the Mo(VI)/Mo(V) couple, MoO3 is of interest in electrochemical devices and displays.[4]
References
- ^ http://www.mallbaker.com/americas/msds/english/m7829_msds_us_default.pdf
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Heynes, J. B. B.; Cruywagen, J. J. "Yellow Molybdenum(VI) Oxide Dihydrate" Inorganic Syntheses, 1986, volume 24, pp. 191. ISBN 0-471-83441-6.
- ^ Ferreira, F. F.; Souza Cruz, T. G.; Fantini, M. C. A.; Tabacniks, M. H.; de Castro, S. C.; Morais, J.; de Siervo, A.; Landers, R.; Gorenstein, A. Solid State Ionics. 2000, 136-137, 357.
External links
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- U.S. Department of Health and Human Services National Toxicology Program
- International Molybdenum Association
- Los Alamos National Laboratory- Molybdenum
 Molybdite on molybdenite, Questa molybdenum mine, New Mexico. Size: 11.0 x 6.7 x 4.1 cm.
Molybdite on molybdenite, Questa molybdenum mine, New Mexico. Size: 11.0 x 6.7 x 4.1 cm.
Molybdenum compounds Categories:- Molybdenum compounds
- Oxides
Wikimedia Foundation. 2010.