- Molybdenum hexafluoride
-
Molybdenum hexafluoride 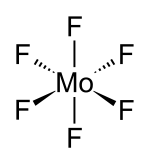
Identifiers CAS number 7783-77-9 
Properties Molecular formula MoF6 Molar mass 209.93 g/mol Appearance white crystals or colorless liquid
hygroscopicDensity 2.54 g/cm3 Melting point 17.5 °C, 291 K, 64 °F
Boiling point 34 °C, 307 K, 93 °F
Solubility in water hydrolyzes Structure Crystal structure Orthorhombic, oP28 Space group Pnma, No. 62 Coordination
geometryoctahedral (Oh) Dipole moment 0  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Molybdenum hexafluoride is the highest fluoride of molybdenum. It is a solid which melts just below room temperature; in water, it hydrolyses to give hydrofluoric acid.[1]
It has few uses, and generally appears as an impurity in uranium hexafluoride (in the nuclear industry) or tungsten hexafluoride (in the semiconductor industry; WF6 is used for chemical vapour deposition of tungsten); it can be removed from the latter by reduction of a WF6-MoF6 mixture with any of a number of elements including molybdenum at moderately elevated temperature.[2][3]
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- ^ US-Patent 5234679: Method of Refining Tungsten Hexafluoride Containing Molybdenum Hexafluoride as an Impurity, 10. August 1993.
- ^ US-Patent 6896866: Method for Purification of Tungsten Hexafluoride, 24. Mai 2005.
Molybdenum compounds Categories:- Molybdenum compounds
- Fluorides
- Octahedral compounds
Wikimedia Foundation. 2010.
