- Delphinidin
-
Delphinidin 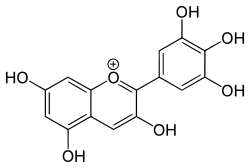 2-(3,4,5-trihydroxyphenyl)chromenylium-3,5,7-triol
2-(3,4,5-trihydroxyphenyl)chromenylium-3,5,7-triolIdentifiers CAS number 13270-61-6, [528-53-0] (chloride) PubChem 68245 ChemSpider 61545 
ChEMBL CHEMBL590878  , CHEMBL276780
, CHEMBL276780Jmol-3D images Image 1
Image 2- Oc3cc(O)cc(c3CC2O)OC2c(cc1O)cc(O)c1O
[Cl-].Oc1cc(cc(O)c1O)c3[o+]c2cc(O)cc(O)c2cc3O
- InChI=1S/C15H10O7.ClH/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6;/h1-5H,(H5-,16,17,18,19,20,21);1H

Key: FFNDMZIBVDSQFI-UHFFFAOYSA-N
InChI=1/C15H10O7.ClH/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6;/h1-5H,(H5-,16,17,18,19,20,21);1H
Key: FFNDMZIBVDSQFI-UHFFFAOYAW
Properties Molecular formula C15H11O7 Molar mass 303.24 g mol−1 Exact mass 303.050477  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant.[1] Delphinidin gives blue hues to flowers like violas and delphiniums. It also gives the blue-red color of the grape that produces Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates.[2]
Delphinidin, like nearly all other anthocyanidins, is pH-sensitive, and changes from blue in basic solution to red in acidic solution.
Glycosides
Myrtillin (Delphinidin-3-O-glucoside) and tulipanin (delphinidin-3-O-rutinoside) can be found in blackcurrant pomace.
Violdelphin (Delphinidin 3-rutinoside-7-O-(6-O-(4-(6-O-(4-hydroxybenzoyl)-beta-D-glucosyl)oxybenzoyl)-beta-D-glucoside) is responsible for purplish blue flower color of Aconitum chinense.[3]References
- ^ AFAQ Farrukh; SYED Deeba N.; MALIK Arshi; HADI Naghma; SARFARAZ Sami; KWEON Mee-Hyang; KHAN Naghma; MOHAMMAD ABU ZAID; MUKHTAR Hasan (2007). "Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis". Journal of investigative dermatology 127 (1): 222–232. doi:10.1038/sj.jid.5700510. PMID 16902416.
- ^ Ribereau-Gayon, Jean; Ribereau-Gayon, Pascal (1958). "The anthocyans and leucoanthocyans of grapes and wines". American Journal of Enology 9: 1–9.
- ^ Violdelphin on PubChem
3-hydroxyanthocyanidins 5-Desoxy-peonidin | Aurantinidin | Cyanidin | 6-Hydroxycyanidin | Delphinidin | Fisetinidin | Guibourtinidin | Pelargonidin | Robinetinidin3-deoxyanthocyanidins Apigeninidin | Columnidin | Diosmetinidin | Gesneridin | Luteolinidin | TricetinidinO-methylated anthocyanidins Anthocyanins Antirrhinin | Chrysanthemin | Malvin | Myrtillin | Oenin | Primulin | Pulchellidin 3-glucoside | Pulchellidin 3-rhamnoside | TulipaninAcetylated glycosides Cyanidin-3-(di-p-coumarylglucoside)-5-glucoside (found in Dark opal basil) Violdelphin (found in Aconitum chinense)Misc. Metalloanthocyanins (Commelinin | Cyanosalvianin | Protocyanin | Protodelphin) | Pyranoanthocyanins | CopigmentationCategories:- Anthocyanidins
- Phenolic compounds in wine
- Oc3cc(O)cc(c3CC2O)OC2c(cc1O)cc(O)c1O
Wikimedia Foundation. 2010.
