- Chrysanthemin
-
Chrysanthemin 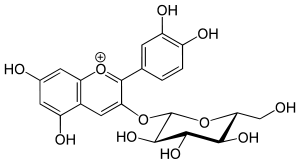 (2S,3R,4S,5S,6R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol chlorideOther namesChrysontenin
(2S,3R,4S,5S,6R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol chlorideOther namesChrysontenin
Glucocyanidin
Asterin
Chrysanthemin
Purple corn color
Kuromanin chloride
Cyanidin 3-glucoside
Cyanidol 3-glucoside
Cyanidine 3-glucoside
Cyanidin 3-O-glucoside
cyanidin-3-O-beta-D-glucoside
Cyanidin 3-monoglucosideIdentifiers CAS number 7084-24-4 
PubChem 197081 ChemSpider 170681 
Jmol-3D images Image 1 - [Cl-].O(c1c([o+]c2c(c1)c(O)cc(O)c2)c3ccc(O)c(O)c3)[C@@H]4O[C@@H]([C@@H](O)[C@H](O)[C@H]4O)CO
- InChI=1S/C21H20O11.ClH/c22-7-16-17(27)18(28)19(29)21(32-16)31-15-6-10-12(25)4-9(23)5-14(10)30-20(15)8-1-2-11(24)13(26)3-8;/h1-6,16-19,21-22,27-29H,7H2,(H3-,23,24,25,26);1H/t16-,17-,18+,19-,21-;/m1./s1

Key: YTMNONATNXDQJF-UBNZBFALSA-N
InChI=1/C21H20O11.ClH/c22-7-16-17(27)18(28)19(29)21(32-16)31-15-6-10-12(25)4-9(23)5-14(10)30-20(15)8-1-2-11(24)13(26)3-8;/h1-6,16-19,21-22,27-29H,7H2,(H3-,23,24,25,26);1H/t16-,17-,18+,19-,21-;/m1./s1
Key: YTMNONATNXDQJF-UBNZBFALBB
Properties Molecular formula C21H21O11+, Cl-
C21H21ClO11Molar mass 484.83 g/mol (chloride)
449.38 g/molExact mass 484.077239 (chloride)
449.108387 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chrysanthemin is an anthocyanin. It is the 3-glucoside of cyanidin. It has been detected in blackcurrant pomace, roselle plant, Japanaese angiosperm,[1] Rhaponticum,[2] victoria plum,[3] and açaí.[4] The biosynthesis of cyanidin 3-O-glucoside in Escherichia coli was demonstrated by mean of metabolic genetic engineering.[5]
References
- ^ A survey of anthocyanins in sprouting leaves of some Japanese angiosperms studies on anthocyanins, LXV. Kunijiro Yoshitama, Makiko Ozaku, Michiko Hujii and Kôzô Hayashi, Journal of plant research, Volume 85, Number 4, pp. 303-306, doi:10.1007/BF02490176
- ^ Chrysanthemin and cyanin in species of the genus Rhaponticum. V. V. Vereskovskii and I. I. Chekalinskaya, Chemistry of natural compounds, Volume 14, Number 4, pp. 450-451, doi:10.1007/BF00565267
- ^ The chemical constituents of victoria plums: Chrysanthemin, acid and pectin contents. D. Dickinson and Joy H. Gawler, Journal of the Science of Food and Agriculture, Volume 7, Issue 11, November 1956, pp. 699–705 doi:10.1002/jsfa.2740071103
- ^ Del Pozo-Insfran D, Brenes CH, Talcott ST (March 2004). "Phytochemical composition and pigment stability of Açai (Euterpe oleracea Mart.)". J. Agric. Food Chem. 52 (6): 1539–45. doi:10.1021/jf035189n. PMID 15030208.
- ^ Yan Y, Chemler J, Huang L, Martens S, Koffas MA (2005). "Metabolic engineering of anthocyanin biosynthesis in Escherichia coli". Appl. Environ. Microbiol. 71 (7): 3617–23. doi:10.1128/AEM.71.7.3617-3623.2005. PMID 16000769.
3-hydroxyanthocyanidins 5-Desoxy-peonidin | Aurantinidin | Cyanidin | 6-Hydroxycyanidin | Delphinidin | Fisetinidin | Guibourtinidin | Pelargonidin | Robinetinidin3-deoxyanthocyanidins Apigeninidin | Columnidin | Diosmetinidin | Gesneridin | Luteolinidin | TricetinidinO-methylated anthocyanidins Anthocyanins Acetylated glycosides Cyanidin-3-(di-p-coumarylglucoside)-5-glucoside (found in Dark opal basil) Violdelphin (found in Aconitum chinense)Misc. Metalloanthocyanins (Commelinin | Cyanosalvianin | Protocyanin | Protodelphin) | Pyranoanthocyanins | CopigmentationCategories:- Anthocyanidin glucosides
- Phenolic compounds in wine
Wikimedia Foundation. 2010.
