- Vinyl fluoride
-
Vinyl fluoride 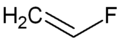 FluoroetheneOther namesVinylfluoride, Fluoroethylene, Monofluoroethylene, Vinyl fluoride monomer, VF, R 1141, UN 1860 (inhibited)
FluoroetheneOther namesVinylfluoride, Fluoroethylene, Monofluoroethylene, Vinyl fluoride monomer, VF, R 1141, UN 1860 (inhibited)Identifiers CAS number 75-02-5 
PubChem 6339 ChemSpider 6099 
EC number 200-832-6 KEGG C19185 
ChEBI CHEBI:51314 
RTECS number YZ7351000 Jmol-3D images Image 1 - FC=C
Properties Molecular formula C2H3F Molar mass 46.04 g/mol Appearance Colorless gas with a faint, ethereal odor Density 2 g/cm3 (gas) 0.91 g/cm3 (liquid)
Melting point -160.5 °C (-257 °F)
Boiling point -72.2 °C (-98 °F)
Solubility in water Slightly soluble Vapor pressure 25 500 kPa Hazards EU classification Extremely flammable (F+) R-phrases R12 S-phrases S9, S16, S33 NFPA 704 Autoignition
temperature385 °C Explosive limits 2.6 - 21.7 %  fluoride (verify) (what is:
fluoride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Vinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint etherlike odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride.
Contents
Production
It was first prepared in 1901 by Frédéric Swarts, the Belgian chemist who was the first to prepare CFCs in 1892. Swarts used the reaction of zinc with 1,1-difluoro-2-bromoethane. It is produced industrially by two routes, one being the mercury-catalyzed reaction of acetylene and hydrogen fluoride:[1]
- HCCH + HF → CH2=CHF
It is also prepared from 1,1-chlorofluoroethane:
- CH3CHClF → CH2=CHF + HCl
Safety
Vinyl fluoride is classified as an IARC Group 2A carcinogen (likely to cause cancer in humans).
See also
References
- ^ Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_349
Additional data
Its critical point is at 54.8 °C (328 K) and 5.24 MPa. Molecular dipole moment is 1.4 Debye and heat of vaporization is 361 kJ/kg.
External links
- US Occupational Safety and Health Administration data sheet
- Vinyl fluoride data sheet, EnvironmentalChemistry.com
- Vinyl fluoride data sheet, airliquide.com
- MSDS Safety data at inchem.org
- Information about its carcinogenity
Categories:- Organofluorides
- Refrigerants
- IARC Group 2A carcinogens
- Chemistry stubs
Wikimedia Foundation. 2010.

