- 1,8-Bis(dimethylamino)naphthalene
-
1,8-Bis(dimethylamino)naphthalene 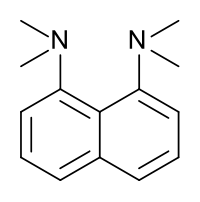
 N,N,N',N'-tetramethylnaphthalene-1,8-diamineOther namesProton Sponge
N,N,N',N'-tetramethylnaphthalene-1,8-diamineOther namesProton SpongeIdentifiers CAS number 20734-58-1 PubChem 88675 ChemSpider 80012 
Jmol-3D images Image 1
Image 2- CN(C)C1=CC=CC2=C1C(=CC=C2)N(C)C
c1(cccc2cccc(N(C)C)c12)N(C)C
Properties Molecular formula C14H18N2 Molar mass 214.31 g mol−1 Melting point 47.8 °C, 321 K, 118 °F
Acidity (pKa) 12.1 (in water)[1]
18.62 (in acetonitrile)[2]
(acidity of the conjugate acid C14H18N2H+) (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,8-Bis(dimethylamino)naphthalene is a chemical compound that was first prepared in 1968[1] by Roger Alder FRS at the University of Bristol. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.[3][4] This compound is a diamine in which the two dimethylamino groups are attached on the same side or peri position of a naphthalene system. Proton-sponge has several very interesting properties; one is its very high basicity; another is its spectroscopic properties.
Contents
Structure and properties
With a pKa of 12.1 [1] for its conjugate acid in aqueous solution, 1,8-bis(dimethylamino)naphthalene is one of the strongest amine bases known, although it only absorbs protons slowly—hence the trade name. The high basicity is attributed to the relief of strain upon protonation and/or the strong interaction between the nitrogen lone pairs.[3][5] However, the molecule is sterically hindered, making it a weak nucleophile. Because of this combination of properties, it has been used in organic synthesis as a highly selective non-nucleophilic base.
The spectroscopic properties of Proton-sponge are very interesting for researchers of molecular chemistry and have been researched for a long time. Proton-sponge emits a double fluorescence in various solutions due to the mixture of two ground-state species.[4]
Preparation
This compound is commercially available; it may be prepared by the methylation of 1,8-diaminonaphthalene with iodomethane or dimethyl sulfate.[6]
Reactions
Proton-sponge is methylated by using dimethyl sulfate. The pKa of trimethylated Proton-sponge is 6.43 in aqueous solution.
Other proton sponges
Second generation proton sponges are known with even higher basicity. 1,8-bis(hexamethyltriaminophosphazenyl)naphthalene or HMPN[7] is prepared from 1,8-diaminonaphthalene by reaction with tris(dimethylamino)bromophosphonium bromide in the presence of triethylamine. HMPN has a pKBH+ of 29.9 in acetonitrile which is more than 11 orders of magnitude higher than Proton Sponge.
References
- ^ a b c R. W. Alder, P. S. Bowman, W. R. S. Steele, and D. R. Winterman (1968). "The remarkable basicity of 1,8-bis(dimethylamino)naphthalene". Chem. Commun. (13): 723. doi:10.1039/C19680000723.
- ^ I. Kaljurand, A. Kütt, L. Sooväli, T. Rodima, V. Mäemets, I. Leito, I. A. Koppel. Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales. J. Org. Chem., 2005, 70, 1019–1028. DOI: 10.1021/jo048252w
- ^ a b F. Gerson, E.Haselbach, G. Plattner (December 1971). "Radical anion of 1,8-bis(dimethylamino)naphthalene ("proton sponge")" (abstract). Chem. Phys. Lett. 12 (2): 316–319. Bibcode 1971CPL....12..316G. doi:10.1016/0009-2614(71)85074-1.
- ^ a b A. Szemik-Hojniak, W.Rettig, I. Deperasinska (2001-08-03). "The forbidden emission of protonated proton sponge". Chem. Phys. Lett. 343 (3): 404–412. doi:10.1016/S0009-2614(01)00690-X.
- ^ R. W. Alder (1989). "Strain effects on amine basicities". Chem. Rev. 89 (5): 1215–1223. doi:10.1021/cr00095a015.
- ^ Sorokin, Vladimir I.; Ozeryanskii, Valery A.; Pozharskii, Alexander F. (2003). "A Simple and Effective Procedure for the N-Permethylation of Amino-Substituted Naphthalenes". European Journal of Organic Chemistry 2003 (3): 496. doi:10.1002/ejoc.200390085.
- ^ Volker Raab, Ekaterina Gauchenova, Alexei Merkoulov, Klaus Harms, Jörg Sundermeyer, Borislav Kovačević, and Zvonimir B. Maksić (2005). "1,8-Bis(hexamethyltriaminophosphazenyl)naphthalene, HMPN: A Superbasic Bisphosphazene "Proton Sponge"". J. Am. Chem. Soc. 127 (45): 15738–15743. doi:10.1021/ja052647v. PMID 16277515.
External links
Categories:- 1-Naphthylamines
- Reagents for organic chemistry
- CN(C)C1=CC=CC2=C1C(=CC=C2)N(C)C
Wikimedia Foundation. 2010.
