- HMX
-
For other uses, see HMX (disambiguation).
HMX 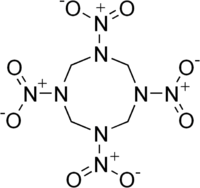
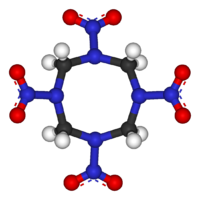 Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocineIdentifiers CAS number 2691-41-0 
PubChem 17596 ChemSpider 16636 
ChEBI CHEBI:33176 
Jmol-3D images Image 1 - C1N(CN(CN(CN1[N+](=O)[O-])[N+](=O)[O-])[N+](=O)[O-])[N+](=O)[O-]
Properties Molecular formula C4H8N8O8 Molar mass 296.155 g/mol Density 1.91 g/cm3, solid Melting point 276-286 °C
Explosive data Shock sensitivity Low Friction sensitivity Low Explosive velocity 9100 m/s RE factor 1.70  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references HMX, also called octogen, is a powerful and relatively insensitive nitroamine high explosive, chemically related to RDX. Like RDX, the name has been variously listed as High Melting eXplosive, Her Majesty's eXplosive, High-velocity Military eXplosive, or High-Molecular-weight rdX.[1]
The molecular structure of HMX consists of an eight-membered ring of alternating carbon and nitrogen atoms, with a nitro group attached to each nitrogen atom. Because of its high molecular weight, it is one of the most potent chemical explosives manufactured, although a number of newer ones, including HNIW and octanitrocubane, are more powerful.
Contents
Production
HMX is more complicated to manufacture than most explosives and this confines it to specialist applications. It may be produced by nitration of hexamine in the presence of acetic anhydride, paraformaldehyde and ammonium nitrate. RDX produced using the Bachmann Process usually contains 8–10% HMX.[2]
Applications
Also known as cyclotetramethylene-tetranitramine, tetrahexamine tetranitramine, or octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, HMX was first made in 1930. In 1949 it was discovered that HMX can be prepared by nitrolysis of RDX. Nitrolysis of RDX is performed by dissolving RDX in a 55% HNO3 solution, followed by placing the solution on a steambath for about six hours.[3] HMX is used almost exclusively in military applications, including as the detonator in nuclear weapons, in the form of polymer-bonded explosive, and as a solid rocket propellant.
During World War II, under the code name Aunt Jemima, HMX was mixed with flour and used by Chinese guerrillas to disrupt the Japanese invasion and occupation of China. The mixture could easily pass for regular flour, thereby passing checkpoints without detection. It could even be cooked into pancakes without exploding and eaten without poisoning anyone. Uneaten pancakes or unused dough could still be used later for its original explosive purposes. In China during WWII, 15 tons of the "Aunt Jemima" HMX mixture was used. None was ever "discovered" by the Japanese. (Source: Weird Warfare episode on History Channel, Air Date 4-6-2011)
HMX is used in melt-castable explosives when mixed with TNT, which as a class are referred to as "octols". Additionally, polymer-bonded explosive compositions containing HMX are used in the manufacture of missile warheads and armor-piercing shaped charges.
HMX is also used in the process of perforating the steel casing in oil and gas wells. The HMX is built into a shaped charge that is detonated within the wellbore to punch a hole through the steel casing and surrounding cement out into the hydrocarbon bearing formations. The pathway that is created allows formation fluids to flow into the wellbore and onward to the surface.
See also
- 2,4,6-Tris(trinitromethyl)-1,3,5-triazine
- 4,4’-Dinitro-3,3’-diazenofuroxan (DDF)
- Heptanitrocubane
- HHTDD
- Octanitrocubane
Notes
- ^ Cooper, Paul W., Explosives Engineering, New York: Wiley-VCH, 1996. ISBN 0-471-18636-8
- ^ http://www.globalsecurity.org/military/systems/munitions/explosives-nitramines.htm
- ^ WE Bachmann, JC Sheehan (1949). "A New Method of Preparing the High Explosive RDX1". Journal of the American Chemical Society, 1949 (5): 1842–1845.
References
- Cooper, Paul W., Explosives Engineering, New York: Wiley-VCH, 1996. ISBN 0-471-18636-8
- Urbanski, Tadeusz. Chemistry and Technology of Explosives. Vol. III., Warszawa: Polish Scientific Publishers, 1967
Categories:- Explosive chemicals
- Nitroamines
- Nitrogen heterocycles
Wikimedia Foundation. 2010.
