- Xenon tetroxide
-
Xenon tetroxide 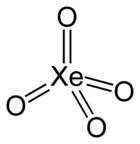
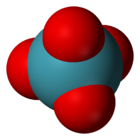 xenon tetraoxide
xenon tetraoxide
xenon(VIII) oxideStructure Molecular shape Tetrahedral[1] Dipole moment 0 D Properties Molecular formula XeO4 Molar mass 195.29 g mol−1 Appearance Yellow solid below −36°C Density ? g cm−3, solid Melting point −35.9 °C
Boiling point 0 °C[2]
Thermochemistry Std enthalpy of
formation ΔfHo298+153.5 kcal mol−1 [3] Standard molar
entropy So298? J.K−1.mol−1 Hazards EU classification Explosive (E) Related compounds Related compounds Perxenic acid
Xenon trioxide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to explode, decomposing into xenon and oxygen (O2).[4][5]
All eight valence shell electrons of xenon are involved in the bonds with the oxygen, and the oxidation state of the xenon atom is +8. Oxygen is the only element that can bring xenon up to its highest oxidation state; even fluorine can only give XeF6.
Reactions
At temperatures above −35.9 °C, xenon tetroxide is very prone to explosion, decomposing into xenon gas and oxygen with ΔH = −643 kJ/mol:
- XeO4 → Xe + 2 O2
The two other short lived xenon compounds with an oxidation state of +8 are accessible by the reaction of xenon tetroxide with xenon hexafluoride. XeO3F2 and XeO2F4 can be detected with mass spectrometry.
Synthesis
All syntheses start from the perxenates, which are accessible from the xenates through two methods. One is the disproportionation of xenates to perxenates and xenon:
- 2 XeO42– → XeO64– + Xe + O2
The other is oxidation of the xenates with ozone in basic solution:
- XeO42– + O3 + 2 OH– → XeO64– + O2 + H2O
Barium perxenate is reacted with sulfuric acid and the unstable perxenic acid is dehydrated to give xenon tetroxide:
- Ba2XeO6 + 2 H2SO4 → 2 BaSO4 + H4XeO6
- H4XeO6 → 2 H2O + XeO4
The unstable perxenic acid slowly undergoes a disproportionation reaction to the xenic acid and oxygen:
- 2 H4XeO6 → O2 + 2 H2XeO4 + 2 H2O
References
- ^ G. Gundersen, K. Hedberg, J. L.Huston (1970). "Molecular Structure of Xenon Tetroxide, XeO4". J. Chem. Phys. 52 (2): 812–815. doi:10.1063/1.1673060.
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 494. ISBN 0-8493-0594-2
- ^ Gunn, S. R. (May 1965). "The Heat of Formation of Xenon Tetroxide". Journal of the American Chemical Society 87 (10): 2290–2291. doi:10.1021/ja01088a038.
- ^ H.Selig , J. G. Malm , H. H. Claassen , C. L. Chernick , J. L. Huston (1964). "Xenon tetroxide -Preparation + Some Properties". Science 143 (3612): 1322–3. doi:10.1126/science.143.3612.1322. JSTOR 1713238. PMID 17799234.
- ^ J. L. Huston, M. H. Studier, E.N. Sloth (1964). "Xenon tetroxide - Mass Spectrum". Science 143 (3611): 1162–3. doi:10.1126/science.143.3611.1161-a. JSTOR 1712675. PMID 17833897.
- Lide, D. R., ed (2002). CRC Handbook of Chemistry and Physics (83rd ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0483-0.
Xenon compounds Categories:- Xenon compounds
- Inorganic compounds
- Oxides
Wikimedia Foundation. 2010.
