- Noble gas compound
-
Contents
Noble gas compounds are chemical compounds that include an element from Group 18 of the periodic table, the noble gases.
History and background
It was initially believed that the noble gases could not form compounds due to their full valence shell of electrons that rendered them very chemically stable and unreactive.
All noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Because of their high ionization energy and almost zero electron affinity, they were not expected to be reactive.
In 1933 Linus Pauling predicted that the heavier noble gases would be able to form compounds with fluorine and oxygen. Specifically, he predicted the existence of krypton hexafluoride and xenon hexafluoride (XeF6), speculated that XeF8 might exist as an unstable compound, and suggested that xenic acid would form perxenate salts.[1][2] These predictions proved quite accurate, although subsequent predictions for XeF8 indicated that it would be not only thermodynamically unstable, but kinematically unstable.[3] As of 2009, XeF8 has not been made, although the octafluoroxenon(VI) anion (XeF82−) has been observed.
The heavier noble gases have more electron shells than the lighter ones. Hence, the outermost electrons experience a shielding effect from the inner electrons that makes them more easily ionized, since they are less strongly attracted to the positively-charged nucleus. This results in an ionization energy low enough to form stable compounds with the most electronegative elements, fluorine and oxygen, and even with less electronegative elements such as nitrogen and carbon under certain circumstances.[4][5]
Pre-1962 compounds
Prior to 1962, the only isolated compounds of noble gases were clathrates (including clathrate hydrates). Other compounds such as coordination compounds were observed only by spectroscopic means.[2]
Clathrates
Clathrates (also known as cage compounds) are compounds of noble gases in which they are trapped within cavities of crystal lattices of certain organic and inorganic substances. The essential condition for their formation is that the guest (noble gas) atoms should be of appropriate size to fit in the cavities of the host crystal lattice. For instance, Ar, Kr, and Xe can form clathrates with β-quinol, but He and Ne cannot fit because they are too small.
Clathrates have been used for separation of He and Ne from Ar, Kr, and Xe, and also for the transportation of Ar, Kr, and Xe. In addition,85Kr clathrate provides a safe source of beta particles, while 133Xe clathrate provides a useful source of gamma rays.
Noble gas atoms such as Kr and Xe can appear as guests in crystals of melanophlogite.
Coordination compounds
Coordination compounds such as Ar·BF3 have been postulated to exist at low temperatures, but have never been confirmed. Also, compounds such as WHe2 and HgHe2 were reported to have been formed by electron bombardment, but recent research has shown that these are probably the result of He being adsorbed on the surface of the metal; therefore, these compounds cannot truly be considered chemical compounds.
Hydrates
Hydrates are formed by compressing the noble gases in water. It is believed that the water molecule, a strong dipole, induces a weak dipole in the noble gas atoms, resulting in dipole-dipole interaction. Heavier atoms are more influenced than smaller ones, hence Xe·6H2O is the most stable hydrate. The existence of these compounds has, however, been disputed in recent years.[citation needed]
True noble gas compounds
Helium compounds
Although there is some theoretical evidence for a few metastable helium compounds which may be stable at very low temperatures and extreme pressures, none are confirmed by experiments.
Neon compounds
A neon compound is still yet to be theoretically identified.
Argon compounds
The discovery of HArF was announced in 2000.[6]
Krypton compounds
Krypton is able to react with fluorine to form KrF2.
Xenon compounds
The first published report, in June 1962, of a noble gas compound was by Neil Bartlett, who noticed that the highly oxidising compound platinum hexafluoride ionised O2 to O2+. As the ionisation energy of O2 to O2+ (1165 kJ mol–1) is nearly equal to the ionisation energy of Xe to Xe+ (1170 kJ mol–1), he tried the reaction of Xe with PtF6. This yielded a crystalline product xenon hexafluoroplatinate, whose formula was proposed to be Xe+[PtF6]−.[2][7] It was later shown that the compound is actually more complex, containing both XeFPtF6 and XeFPt2F11.[8] This was the first real compound of any noble gas.
In September 1962, Howard Claasen reported the synthesis of a simple (two-element) noble gas compound, xenon tetrafluoride, by subjecting xenon and fluorine to a high temperature.[9] In November 1962, Rudolf Hoppe of Universität Münster reported that xenon and fluorine can react to form xenon difluoride.[10]
In recent years, several compounds of noble gases, particularly xenon, have been prepared. Among these are the xenon fluorides (XeF2, XeF4, XeF6), oxyfluorides (XeOF2, XeOF4, XeO2F2, XeO3F2, XeO2F4) and oxides (XeO3 and XeO4). Xenon difluoride can be produced by the simple exposure of Xe and F2 gases to sunlight; while the mixing of the two gases had been tried over 50 years before in an attempt to produce a reaction, nobody had thought to simply expose the mixture to sunlight.
Xenon fluorides react with several fluorides to form fluoroxenates, such as sodium octafluoroxenate (Na+2XeF82-), and fluoroxenonium salts, such as trifluoroxenonium hexafluoroantimoniate (XeF3+SbF6-).
Recently xenon has been shown to produce a wide variety of compounds of the type XeOnX2 where n is 1,2 or 3 and X is any electronegative group, such as CF3, C(SO2CF3)3, N(SO2F)2, N(SO2CF3)2, OTeF5, O(IO2F2), etc. The range of compounds is impressive, running into the thousands and involving bonds between xenon and oxygen, nitrogen, carbon, boron and even gold, as well as perxenic acid, several halides, and complex ions; a range of compounds similar to that seen with the neighbouring element iodine. The compound Xe2Sb2F11 contains a Xe–Xe bond, the longest element-element bond known (308.71 pm = 3.0871 Å).[11]
Short-lived excimers of Xe2 and noble gas halides such as XeCl2 are used in excimer lasers.
Radon compounds
Radon reacts with fluorine to form RnF2, which glows with a yellow light in the solid state.
Fullerene compounds
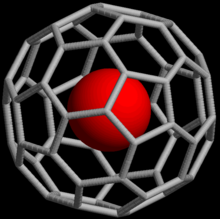
Noble gases can also form endohedral fullerene compounds where the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when C60 is exposed to a pressure of around 3 bar of He or Ne, the complexes He@C60 and Ne@C60 are formed.[12] Under these conditions, only about one out of every 650,000 C60 cages was doped with a helium atom; with higher pressures (3000 bar), it is possible to achieve a yield of up to 0.1%. Endohedral complexes with argon, krypton and xenon have also been obtained, as well as numerous adducts of He@C60.[13]
Applications
Most applications of noble gas compounds are either as oxidising agents or as a means to store noble gases in a dense form. Xenic acid is a valuable oxidising agent because it has no potential for introducing impurities: the xenon is simply liberated as a gas. It is rivalled only by ozone in this respect.[2] The perxenates are even more powerful oxidising agents, and the xenon fluorides are good fluorinating agents. Stable salts of xenon containing very high proportions of fluorine by weight such as perfluoroammonium heptafluoroxenon, NF4XeF7 and the related (NF4)2XeF8 have been developed as highly energetic oxidisers for use as propellants in rocketry.[14][15]
Xenon-based compounds have also been used for synthesizing carbocations stable at room temperature in SO2ClF solution.[16]
Radioactive isotopes of krypton and xenon are difficult to store and dispose, and compounds of these elements may be more easily handled than the gaseous forms.[2]
References
- ^ Linus Pauling (June 1933). "The Formulas of Antimonic Acid and the Antimonates". J. Am. Chem. Soc. 55 (5): 1895–1900. doi:10.1021/ja01332a016.
- ^ a b c d e Holloway, John H. (1968). Noble-Gas Chemistry. London: Methuen. ISBN 0416032702.
- ^ Seppelt, Konrad (June 1979). "Recent developments in the Chemistry of Some Electronegative Elements". Accounts of Chemical Research 12 (6): 211–216. doi:10.1021/ar50138a004.
- ^ Smith GL, Mercier HP, Schrobilgen GJ (February 2007). "Synthesis of [F3S≡NXeF][AsF6] and structural study by multi-NMR and Raman spectroscopy, electronic structure calculations, and X-ray crystallography". Inorganic Chemistry 46 (4): 1369–78. doi:10.1021/ic061899. PMID 17256847.
- ^ Smith GL, Mercier HP, Schrobilgen GJ (May 2008). "F5SN(H)Xe+; a rare example of xenon bonded to sp3-hybridized nitrogen; synthesis and structural characterization of [F5SN(H)Xe][AsF6]". Inorganic Chemistry 47 (10): 4173–84. doi:10.1021/ic702039f. PMID 18407626.
- ^ Khriachtchev, L., Pettersson, M., Runeberg, N., Lundell, J., Räsänen, M. (2000). "A stable argon compound". Nature 406 (6798): 874–876. doi:10.1038/35022551. PMID 10972285.
- ^ Bartlett, N. (1962). "Xenon hexafluoroplatinate Xe+[PtF6]–". Proceedings of the Chemical Society of London (6): 218. doi:10.1039/PS9620000197.
- ^ Graham, L.; Graudejus, O., Jha N.K., and Bartlett, N. (2000). "Concerning the nature of XePtF6". Coordination Chemistry Reviews 197: 321–334. doi:10.1016/S0010-8545(99)00190-3.
- ^ Claassen, H. H.; Selig, H.; Malm, J. G. (1962). "Xenon Tetrafluoride". J. Am. Chem. Soc. 84 (18): 3593. doi:10.1021/ja00877a042.
- ^ Hoppe, R. ; Daehne, W. ; Mattauch, H. ; Roedder, K. (1962-11-01). "FLUORINATION OF XENON". Angew. Chem. Intern. Ed. Engl.; Vol: 1 1 (11): 599. doi:10.1002/anie.196205992.
- ^ Li, Wai-Kee; Zhou,, Gong-Du; Mak, Thomas C. W. (2008). Advanced Structural Inorganic Chemistry. Oxford University Press. p. 674. ISBN 0199216940.
- ^ M. Saunders, H. A. Jiménez-Vázquez, R. J. Cross, and R. J. Poreda (1993). "Stable compounds of helium and neon. He@C60 and Ne@C60". Science 259 (5100): 1428–1430. doi:10.1126/science.259.5100.1428. PMID 17801275.
- ^ Martin Saunders, Hugo A. Jimenez-Vazquez, R. James Cross, Stanley Mroczkowski, Michael L. Gross, Daryl E. Giblin, and Robert J. Poreda (1994). "Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high pressure". J. Am. Chem. Soc. 116 (5): 2193–2194. doi:10.1021/ja00084a089.
- ^ Christe KO, Wilson WW. Perfluoroammonium and alkali-metal salts of the heptafluoroxenon(VI) and octafluoroxenon(VI) anions. Inorganic Chemistry. 1982 Dec;21(12):4113–4117. doi:10.1021/ic00142a001
- ^ Karl O. Christe, William W. Wilson. Perfluoroammonium salt of heptafluoroxenon anion. US Patent 4428913, Jun 24, 1982
- ^ Hélène P. A. Mercier; Matthew D. Moran; Gary J. Schrobilgen; Christoph Steinberg; Reijo J. Suontamo (2004). "The Syntheses of Carbocations by Use of the Noble-Gas Oxidant, [XeOTeF5][Sb(OTeF5)6]: The Syntheses and Characterization of the CX+
3 (X = Cl, Br, OTeF5) and CBr(OTeF5)+
2 Cations and Theoretical Studies of CX+
3 and BX3 (X = F, Cl, Br, I, OTeF5)". J. Am. Chem. Soc. 126 (17): 5533–5548. doi:10.1021/ja030649e. PMID 15113225.
Resources
- http://www.chemsoc.org/exemplarchem/entries/2001/robson/raregascompounds.htm
- http://www.webelements.com/webelements/elements/
- . doi:10.1002/0471238961.031513161903081.
- Khriachtchev, Leonid; Räsänen, Markku; Gerber, R. Benny (2009). "Noble-Gas Hydrides: New Chemistry at Low Temperatures". Accounts of Chemical Research 42 (1): 183. doi:10.1021/ar800110q. PMID 18720951.
Categories:- Noble gas compounds
Wikimedia Foundation. 2010.