- Mammomonogamus
-
Mammomonogamus Scientific classification Kingdom: Animalia Phylum: Nematoda Class: Secernentea Order: Strongylida Family: Syngamidae Genus: Mammomonogamus Species: M. laryngeus Binomial name Mammomonogamus laryngeus
Ryzhikovk, 1948Mammomonogamus is a genus of parasitic nematode of the family Syngamidae that parasitises the respiratory tract of cattle, sheep, goats, deer, cats, orangutans, and elephants. The nematode can also infect humans and cause the disease called Mammomonogamiasis[1]. There are several known species that fall under the Mammomonogamus genus, but the most common species found to infest humans is Mammomonogamus laryngeus. Infection in humans is very rare with only about 100 reported cases worldwide and assumed to be largely accidental[2]. Cases have been reported from the Caribbean[3], China[4], Korea[5], Thailand[6], and Philippines[7]. The worm usually inhabits the upper-respiratory region in the trachea, bronchus, or larynx and can elicit chronic coughing and asthma-like symptoms[8]. One interesting case from Thailand reported finding worms in the patient's duodenum, suggesting that M. laryngeus can also be a gastrointestinal parasite[6]. More research is needed because the life cycle is not completely known. Diagnosis is made by recovering the worms on bronchoscopy or oesophagogastroduodenoscopy. Due to the scant amount of information available on this parasite in literature, increased awareness is necessary especially in endemic areas near M. laryngeus’ reservoir hosts in order to for clinicians, the local population in the endemic area, and traveling tourists to effectively recognize and prevent against Mammomonogamiasis.
Contents
Taxonomic classification
Classification of Mammomonogamus falls under the Syngamidae family. Syngamidae is in the Strongyloidae superfamily and Strongylata order, making them close relatives to hookworms and other nematodes[6].
The genus name Mammomonogamus is derived from the Latin root “Mammo-” (breast) and the Greek roots “mono” (single) and “gamus” (marriage)[9], which most likely is referring to the distinct characteristic of the male and female worm acting as a single unit through the male being joined in permanent copulation to the middle portion of the female’s body.
Species within this genus are Mammomonogamus laryngeus, Mammomonogamus nasicola, and Mammomonogamus gangguiensis[2]. Only M. laryngeus is known to infect and cause disease in humans[6]. Because of M. laryngeus’ close resemblance to the gapeworm from the Syngamus genus that commonly infect birds, M. laryngeus was originally called Syngamus laryngeus and Syngamus kingi[6]. The classification was revised in 1948 when Ryzhikov reconstructed the phylogenetic relationship of the Syngamidae family and re-categorized the parasite as M. laryngeus.[10]
Infestation with M. laryngeus has been called Mammomonogamiasis, Mammomonogamosis, Syngamosis, or Syngamiasis[11]
History
The Mammomonogamus parasite of greatest significance to humans is M. laryngeus due to the occasional, incidental cases reported in humans. While M. laryngeus commonly infects domestic ungulates and ruminants, the first reported case of a human infection was by Dr. King who diagnosed the parasite in a woman from St. Lucia, Antilles[3]. The next case originated in Brazil in the 1920s where the connection with bovine species was confirmed[12].
Morphology
The most distinct feature of M. laryngeus is the “Y” shape that is formed when the male is joined to the female in copula[13]. The smaller male uses its posterior bursa to attach to the female vulva located on the side near the middle of the female worm[14]. The adult worms usually remain permanently joined in this “Y” formation as they settle on the mucosal epithelium of the larynx, trachea, or bronchi[13].
Adult M. laryngeus worms are red to reddish-brown in color due to their hemophagous nature[5][9][13]. They possess spicules ranging from 23-30μm in length and cup-shaped buccal capsules (mouth) that open at the anterior end[2][13][14]. Located deep in the buccal cavity are 8 to 10 teeth that are not thought to be used for attachment[1].
The adult male is about half the length of the female. Case reports have found male worms ranging from 3-6.3mm in length and 360-380μm in width. The larger females were reported to be 8.7-23.5mm long and 550-570μm wide[2][13]. Females also have a pointed posterior end with long or short tails. The female while in copula lays many ellipsoid-shaped eggs that are about 40 x 80μm in size, non-operculated, and usually possess thicker shells than hookworm eggs[5][14].
Life cycle
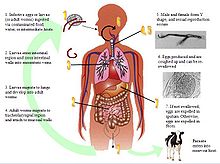
Although the complete life cycle of M. laryngeus is not fully known due to the rareness of the parasite in humans, some have postulated that the parasite adopts a life cycle similar to Syngamus trachea[15], the common gapeworm infection in birds that was initially thought to be Mammomonogamiasis. Currently, there are two existing hypotheses that will help aid medical diagnostics, especially in endemic areas such as the tropics, Caribbean, and Brazil.
Hypothesis #1: Infection initially begins by the ingestion of foods, water, or intermediate hosts contaminated by adult worms. The infective adults migrate to the larynx or trachea and attach to the mucosal walls. Sexual reproduction occurs here, and the females begin to lay eggs in the upper respiratory region. Eggs do not develop at body temperature and are expelled in sputum or re-swallowed and excreted in feces[5].
Hypothesis #2: The infective agent may be embryonated eggs or infective larvae, and infection is due to ingestion of contaminated food, water, or intermediate hosts. As larvae are released into the intestinal area, they can burrow through intestinal walls, travel into the mesenteric veins, and migrate to the alveoli. Here, they undergo a pulmonary cycle, where the larvae develop into adult worms in a process that may take 7 days. After reaching adulthood, M. laryngeus migrates upwards to the trachea, larynx, or bronchi, where sexual reproduction occurs. Egg production begins approximately 3 weeks later, and eggs are coughed up and expelled in sputum or feces. Larvae may hatch from embryonated eggs outside of the mammalian host[5][16].
More research is needed to fully elucidate the life cycle, but it may appear that both larvae and adults can be infective. One recent case reported finding adult worms in the duodenum[6], which is the first presentation of adult worms not in the upper respiratory region. It is possible that the adult worms might have been coughed up and re-swallowed before settling in the duodenum. The development from larvae to adult is about 3 weeks[8], but there is uncertainty regarding the existence of a larval pulmonary cycle. Intermediate hosts, although not fully known, may be earthworms (an intermediate host for the genus Syngamus, the parasite causing avian gapeworm)[14], snails, or arthropods[13]. Other than intermediate hosts, there has been no mention of other biological or mechanical vectors.
Symptoms of infection in humans
Symptoms usually began to appear 6–11 days after initial infection, beginning with a fever and cough. Most cases reported a progression to a persistent cough, leading to expectoration and sometimes hemoptysis[2][13]. Worms in the bronchial region can trigger a chronic, nonproductive cough and asthma-like symptoms due to the obstruction of airways by the worms[2][9][15]. These symptoms along with a low-grade fever can last for several months if not initially diagnosed correctly. A scratching or crawling sensation can be felt in the throat if the worms are attached in the larynx[8][13][15]. Weight loss[17] and pneumonitis[15] have been reported as possible long-term consequences, but not anemia.
Recently, M. laryngeus worms were found in the duodenum of a Thai patient, which was the first gastrointestinal case of Mammomonogamiasis. The patient complained of chest pain, haematemesia, melaena, abdominal bloating, but no respiratory symptoms. Although nothing conclusive was determined, it is possible that the adult worms were dislodged from the larynx, re-swallowed, and later found in the duodenum[6].
Pathogenesis
Little is known about how M. laryngeus causes disease. Symptoms do not arise until the worms have reached the adult stage and obstruct the bronchial airways leading to asthma-like symptoms and coughing[8]. Similar symptoms are seen in humans as well as the domestic ungulates and ruminant hosts. Bronchial inflammation or hemotypsis may occur due to the worms attaching to the mucosal walls and ingesting red blood cells[13].
The incubation period is usually 6–11 days after infection[8]. This supports the second hypothesis of a possible pulmonary cycle that explains the one to two week delay in the presentation of symptoms.
Eosinophilia count is not a reliable measure of extent of infection because it varies from individual to individual. Some cases with multiple pairs of worms have reported low eosinophils levels while other cases with a single pair had very high eosinophil count. Such variation may be due to the lack of host tissue invasion by the parasite, since M. laryngeus attaches to the mucosal epithelium in the tracheolaryngeal region[2]. A singular case even found worms within a cyst[18].
Past cases have demonstrated that simple removal of the worms from upper-respiratory area led to a cessation of symptoms and was a sufficient cure with or without anti-helmintics[2][13]. No lasting pathological tissue damage was reported.
Thus far, there has been no mention of a re-infection mechanism for M. laryngeus, so all adult worms found inside a human must be the result of an ingested embryonated egg, larvae, or adult worm. Most of the time, only one pair of worms is found, but occasionally, the patient may have multiple pairs that must all be removed[2][19].
Diagnosis
The definitive diagnosis is the obtainment of adult worms either by coughing them up[9] or removing them with forceps, a bronchoscope, or endoscopic instruments. However, worms might be difficult to remove if firmly attached to the bronchial walls[5]. M. laryngeus eggs found in the sputum or feces[2][13] is another sure sign of infection.
The eggs closely resemble hookworm eggs, but Mammomonogamus eggs have a much thicker shell[14]. Due to the individual variation on eosinophilia counts, this is not a reliable diagnostic tool[2].
Treatment
Mammomonogamiasis is relatively easy to treat. Manual or bronchoscopic removal of worms has been successful[13]. One case followed up with aspiration[5]. Although no controlled study on the efficacy of anti-helmintics in treating Mammomonogamiasis has been conducted, most patients were given Albendazole, Mebendazole or Thiabendazole with no adverse effects. Patients given Albendazole were instructed to take 400 mg for 3 days[5] or if given a combination of drugs, Albendazole is given 200 mg, 3x a day for 3 days with Mebendazole at 100 mg, 3x a day for 3 days[13]. The drug regimens ranged from 200–3000 mg/day for 3–20 days[2].
Epidemiology
Mammomonogamiasis is a very rare human infection yet a common veterinary parasite. Only 100 human cases of M. laryngeus have been reported thus far[2]. Its reservoir hosts are largely in tropical regions, most commonly the domestic cattle[20], cats[1], orangutans[21], and other ruminants and ungulates. Therefore, humans are accidental hosts[13], where infections are most likely to due close exposure to bovine or feline species.
While the complete life cycle is still not fully known, transmission is thought to be oral-fecal, where infection comes from ingesting contaminated food or water containing embryonated eggs, hatched larvae, or intermediate hosts. Possible intermediate host candidates include earthworms, snails, and arthropods[13]. Eggs are expelled in sputum or feces. Most often, travelers to tropical climate places become exposed to contaminated sources and are diagnosed upon return to their country. The best preventative measure is to ensure proper food preparation and water sanitation.
From the numerous case reports, endemic areas include Martinique, Brazil, Puerto Rico, Dominica, Santa Lucia, Trinidad, Guyana, Guadeloupe[14], India, tropic regions in Africa, Malaysia[19], Philippines[7][22], Vietnam[16], China[23][24], Korea[5], and Thailand[6][19][25].
Due to the frequency of travelers contracting this disease, countries such as Australia[17], Canada[26], United States[2][8][18], UK[27][28][28], France[29][30] have reported cases although these countries are not considered to be endemic for the parasite.
Public health
Mammomonogamiasis is not considered an emerging disease[13] because of the rarity of cases. Consequently, there are no control measures currently being implemented. Very little information about this disease can be found in literature, so physicians especially in endemic areas should be aware of the parasitic disease, the clinical presentations, and treatment for humans[6].
References
- ^ a b c Anderson RC, Chabaud AG, Willmott S. CIH keys to the nematode parasites of vertebrates, no 7. Keys to genera of superfamily Strongyloidea. Commonwealth Agricultural Bureaux, England, 1980.
- ^ a b c d e f g h i j k l m n Nosanchuk, J.S., Wade, S.E., and Landolf, M (1995). Case Report of and Description of Parasite in Mammomonogamus laryngeus (Human Syngamosis) Infection. J of Clinical Microbiology. 33: 998–1000.
- ^ a b Leiper RJ (1913). "Gapes in man, an occasional helminthic infection: a notice of its discovery by Dr A King in St Lucia". Lancet i (4664): 170. doi:10.1016/S0140-6736(00)76203-9.
- ^ Li D, Li G, Zhan X (1997). "Three cases of human Mammomonogamus laryngeus infection". Chin J Parasitol Parasit Dis 15: 281–4.
- ^ a b c d e f g h i Kim HY, Lee SM, Joo JE, et al. (1998). "Human syngamosis: the first case in Korea". Thorax 53 (8): 717–18. doi:10.1136/thx.53.8.717. PMC 1745283. PMID 9828862. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1745283.
- ^ a b c d e f g h i Eamsobhana P, Mongkolporn T, Punthuprapasa P, Yoolek A (3006). "Mammomonogamus roundworm (Nematoda: Syngamidae) recovered from the duodenum of a Thai patient: a first and unusual case originating in Thailand". Trans R Soc Trop Med Hyg 100 (4): 387–91. doi:10.1016/j.trstmh.2005.05.018. PMID 16257022.
- ^ a b SaintJohn JH, Simmons JS, Gardner LL (1929). "Infestation of the lung by a nematode of the genus Cyathostoma". J Am Med Assoc 92: 1816–18.
- ^ a b c d e f Weinstein, L., and A. Molovi. 1971. Syngamus laryngeus infection (Syngamosis) with chronic cough. Ann. Intern. Med. 74:577–580.
- ^ a b c d de Lara, T. de A., Barbosa, M.A., de Oliveira, M.R., de Godoy, I., and Queluz, T.T. (1993). Human syngamosis: Two cases of chronic cough caused by Mammomonogamus laryngeus. Chest. 103(1): 264-5.
- ^ Ryzhikov, K. M. (1948). "[Phylogenetic interrelationships of nematodes of the family Syngamidae and an attempt to reconstruct their systematics]". Doklady Akademii Nauk SSSR 62: 733.
- ^ [1], Mammomonogamiasis. GIDEON Infectious Diseases: Diseases. Accessed 5 Feb. 2009.
- ^ Freitas, A.L., De Carli, G., and Blankenhein, M.H. “Mammonomonogamus (Syngamus) laryngeus infection: a new Brazilian human case. Rev. Inst. Med. Trop. S. Paulo, 37 (2): 177-179, 1995.
- ^ a b c d e f g h i j k l m n o p [www.cdc.gov/eid] Costa, J.C., Delgado, M.L., Vieira, P., Afonso, A., Conde, B., and Cross, J.H (2005). Syngamoniasis in Tourist. Emerging Infectious Diseases. Vol 11, No. 12. .
- ^ a b c d e f Gutierrez, Yezid. Diagnostic Pathology of Parasitic Infections with Clinical Correlations. 2nd ed. New York: Oxford University Press, 2000.
- ^ a b c d Severo LC, Conci LMA, Camargo JJP, Andre-Alves MR, Palombini BC. Syngamosis: two new Brazilian cases and evidence of possible pulmonary cycle. Trans R Soc Trop Med Hyg. 1988;82: 467–8.
- ^ a b Acha PN, Szyfres B. Mammomonogamiasis. Zoonosis and communicable diseases common to man and animals. Washington (DC): Pan American Health Organization; 2003. Scientific and Technical Publication No. 580.
- ^ a b Birrell, D.J., Moorhouse, D.E., Gardnert, M.A.H., and May, C.S (1978). Chronic Cough and Haemoptysis due to a Nematode, "Syngamus Laryngeus." Aust. N.Z. J. Med. 8: 168-170.
- ^ a b Gardiner, C.H. and Schantz, P.M (1983). Mammomonogamus Infection in A Human: Report of A Case. Am. J. Trop. Med. Hyg. 32(5) 995-997.
- ^ a b c Pipitogool V, Chaisiri K, Visetsuspakarn P, Srigan V, Maleewong W. Mammomonogamus (syngamus) laryngeus. First case report in Thailand. Southeast Asian J Trop Med Public Health. 1992;23:336–7.
- ^ Van Aken, D., Lagapa, J.T., Dargantes, A.P., Vercruysse, J., 1996. Mammomonogamus laryngeus (Railliet, 1899) infections in cattle in Mindanao. Philippines. Vet. Parasitol. 64, 329—332.
- ^ Collet, J.Y., Galdikas, B.M., Sugarjito, J., Jojosudharmo, S., 1986. A coprological study of parasitism in orangutans (Pongo pygmaeus) in Indonesia. J. Med. Primatol. 15, 121—129.
- ^ Beaver PC, Jung RC, Wayne E. Clinical parasitology. Philadelphia: Lea and Febiger; 1984.
- ^ Li D, Li G, Zhan X (1997). Three cases of human Mammomonogamus laryngeus infection. Chin J Parasitol Parasit Dis 15: 281–4.
- ^ Qu, F.Y., 1997. First Mammomonogamus laryngeus infection case occurred in Shanghai. Chin. J. Parasitol. Parasit. Dis. 15, 198—200
- ^ Limawongpranee, S., Samanthai, S., Yoolek, A., 2004. Human Mammomonogamus laryngeus infection (syngamosis): the second case report in Thailand. Siriraj Hosp. Gaz. 56, 82—86.
- ^ Leers, W.-D., M. K. Sarin, and K. Arthurs. 1985. Syngamosis, an unusual cause of asthma: the first reported case in Canada. Can. Med. Assoc. J. 132:269–270.
- ^ Basden, R. D. E., J. W. Jackson, and E. I. Jones. 1974. Gapeworm infestation in man. Br. J. Dis. Chest 68:207–209.
- ^ a b Turner P, Turner CG, Bowers KM, Gibson DI, Chiodini PL (2003). A Case of Human Syngamosis. Travel Med Infect Dis. 1(4): 231-3.
- ^ Junod, C., M. Philbert, and H. T. Sang. 1970. Une observation de syngamose humaine a localization bronchique. Premier cas tracte et queri par le thiabendazole. Bull. Soc. Pathol. Exot. 63:483–488.
- ^ Sang, H. T., C. Junod, and M. Philbert. 1970. Notes parasitologiques sur Syngamus laryngeus Railliet, 1899 et la syngamose humaine. A propos d’un cas de syngamose bronchique chez l’homme. Bull. Soc. Pathol. Exot. 63:488–497.
External links
Categories:- Nematodes
- Zoonoses
Wikimedia Foundation. 2010.