- Daidzein
-
Not to be confused with Daidzin.
Daidzein 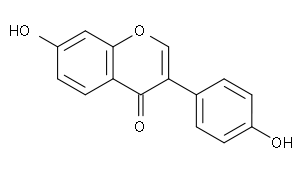 7-Hydroxy-3-(4-hydroxyphenyl) chromen-4-oneOther names4',7-Dihydroxyisoflavone
7-Hydroxy-3-(4-hydroxyphenyl) chromen-4-oneOther names4',7-Dihydroxyisoflavone
Daidzeol
IsoaurostatinIdentifiers CAS number 486-66-8 
PubChem 5281708 ChemSpider 4445025 
UNII 6287WC5J2L 
KEGG C10208 
ChEBI CHEBI:28197 
ChEMBL CHEMBL8145 
Jmol-3D images Image 1 - O=C\1c3c(O/C=C/1c2ccc(O)cc2)cc(O)cc3
Properties Molecular formula C15H10O4 Molar mass 254.23 g/mol Exact mass 254.057909 Appearance Pale yellow prisms Melting point 315-323 °C (decomposes)
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Daidzein belongs to the group of isoflavones. Daidzein and other isoflavone compounds, such as genistein, are present in a number of plants and herbs like the Thai Kwao Krua or Pueraria mirifica, Kudzu or Pueraria lobata, and in food sources such as soybeans and soy products like tofu and textured vegetable protein. Soy isoflavones are a group of compounds found in and isolated from the soybean. Besides functioning as antioxidants, many isoflavones have been shown to interact with animal and human estrogen receptors, and are therefore known as phytoestrogens. Soy isoflavones also produce non-hormonal effects.
Isoflavones act as antioxidants to counteract damaging effects of free radicals in tissues. Isoflavones can act like estrogen in stimulating development and maintenance of female characteristics or they can block cells from using other forms of estrogen[citation needed]. Isoflavones also have been found to have antiangiogenic effects (blocking formation of new blood vessels)[citation needed], and may block the uncontrolled cell growth associated with cancer, most likely by inhibiting the activity of substances in the body that regulate cell division and cell survival (growth factors)[citation needed].[1] Laboratory studies using animals models have shown that both soy and isoflavones might be protective against cancer when given during early life but might stimulate response to cancer-causing chemicals when given during fetal development or when circulating levels of estrogen are low (menopause)[citation needed].
Daidzein is not a drug. It has not been tested in clinical trials to make sure that it is both safe and effective for treating any disease. Daidzein is available as a dietary supplement. Dietary supplements are not drugs, and the labelling of dietary supplements cannot describe the supplement as having any drug activity or effectiveness.
Contents
Biological activities
Scientists have studied some of the activities of daidzein in their laboratories, working with cells or with animals such as mice. Studies in cells and in animals sometimes give hints as to what a chemical might do when given to humans, but no one can know what a chemical does in humans until the chemical is tested in a clinical trial.
Activation of PPARs
Isoflavone daidzein transactivates all three PPAR isoforms, α, δ, and γ and influences target cells.[2] Similar to another isoflavone genistein,[3] daidzein at concentrations between 1 and 100 uM activates PPARs in a dose dependent way in KS483 mesenchymal progenitor cells, breast cancer MCF-7 cells, T47D cells and MDA-MD-231 cells. Studies have shown that both ERs and PPARs influence each other and therefore induce differential effects in a dose-dependent way. The final biological effects of daidzein are determined by the balance among these pleiotrophic actions.[2][4]
Cell proliferation studies
Daidzein, like other isoflavones, has both estrogenic and anti-estrogenic effects. Soy supplements are often consumed by women for alleviating menopausal symptoms or for the perceived protective effects against breast cancer, particularly after a well documented low incidence rates of breast cancer and high soy food intake among Asian women.[5] But taking soy supplements carries serious risks of harm, especially in people who already have cancer and are taking drugs to treat the cancer. Experimental evidence in cells and in animals show that even low concentration stimulates breast tumor growth in in vitro and in vivo, and interfere with the antitumor effect of the cancer drug, tamoxifen.[6] T47D:A18/PKC alpha tumor growth was demonstrated to be stimulated by genistein, but partially inhibited by daidzein; however, coadministration of TAM with either daidzein or genistein produced tumors of greater size.[7] On the other hand an epidemiological study suggests a hypothesis that soy isoflavones consumed at levels comparable to those in Asian populations may reduce the risk of cancer recurrence in women receiving TAM therapy and moreover, appears not to interfere with the drug efficacy.[8] Supportingly daidzein was demonstrated to induce human MCF-7 breast cancer cell line apoptosis through the mitochondrial caspase-dependent cell death pathway.[9]
Antioxidant
Phytoestrogens are often strong antioxidant compounds. Antioxidants are important in maintaining normal physiology. Some people think that antioxidants might prevent aging by eliminating harmful free radicals but there is no evidence that this is true in humans. Scientific studies of daidzein's antioxidant abilities have given contradictory results: some studies have shown antioxidant properties in laboratory experiments on cells, but in other experiments daidzein has caused oxidative stress on cells.[10]
Glycosides
List of plants that contain the chemical
Notes and references
- ^ Merritt RJ, Jenks BH (May 2004). "Safety of soy-based infant formulas containing isoflavones: the clinical evidence". J. Nutr. 134 (5): 1220S–1224S. PMID 15113975. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=15113975.
- ^ a b Dang Z.C. and Löwik C. The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. Journal of Bone and Mineral Research. 2004, 19, 853-861
- ^ Dang Z.C. Dose-dependent effects of soy phyto-oestrogen genistein on adipocytes: mechanisms of action. Obesity Review. 2009, 10, 342-349
- ^ Dang Z.C. and Löwik C. Dose-dependent effects of phytoestrogens on bone: molecular mechanisms, review paper, Trends in Endocrinology and Metabolism. 2005, 16, 207-213
- ^ Messina M, Wu AH. (2009). "Perspectives on the soy-breast cancer relation.". Am J Clin Nutr. 89 (5): 1673S–1679S. doi:10.3945/ajcn.2009.26736V. PMID 19339397. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=19339397.
- ^ de Lemos ML. (2001). "Effects of soy phytoestrogens genistein and daidzein on breast cancer growth.". Ann Pharmacother. 35 (9): 11118–11121. doi:10.1345/aph.10257. PMID 11573864. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=11573864.
- ^ Tonetti DA, Zhang Y, Zhao H, Lim SB, Constantinou AI. (2007). "The effect of the phytoestrogens genistein, daidzein, and equol on the growth of tamoxifen-resistant T47D/PKC alpha.". Nutr Cancer. 58 (2): 1222–1229. doi:10.1080/01635580701328545. PMID 17640169.
- ^ Guha N, Kwan ML, Quesenberry CP Jr, Weltzien EK, Castillo AL, Caan BJ. (2009). "Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study.". Breast Cancer Res Treat. 118 (2): 395–405. doi:10.1007/s10549-009-0321-5. PMID 19221874.
- ^ Jin S, Zhang QY, Kang XM, Wang JX, Zhao WH. (2010). "Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway.". Ann Oncol. 21 (2): 263–268. doi:10.1093/annonc/mdp499. PMID 19889614. http://annonc.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=19889614.
- ^ Röhrdanz E, Ohler S, Tran-Thi QH, Kahl R. (2002). "The phytoestrogen daidzein affects the antioxidant enzyme system of rat hepatoma H4IIE cells.". J Nutr. 132 (2): 370–375. PMID 11880557. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=11880557.
- ^ Chen, Gang et al.; Zhang, J; Ye, J (2001). "Determination of puerarin, daidzein and rutin in Pueraria lobata (Wild.) Ohwi by capillary electrophoresis with electrochemical detection". Journal of Chromatography A (Elsevier) 923 (1 - 2): 255–262. doi:10.1016/S0021-9673(01)00996-7. PMID 11510548.
- ^ Xu, Hua-Neng; Chao-Hong He (2007). "Extraction of isoflavones from stem of Pueraria lobata (Willd.) Ohwi using n-butanol/water two-phase solvent system and separation of daidzein". Separation and Purification Technology (Elsevier) 56 (1): 255–262. doi:10.1016/j.seppur.2007.01.027.
- ^ Zhou, H. Y. et al.. "Separation and determination of puerarin, daidzin and daidzein in stems and leaves of Pueraria thomsonii by RP-HPLC". http://www.ncbi.nlm.nih.gov/pubmed/17655152. Retrieved 29 January 2010.
Isoflavones O-methylated isoflavones Biochanin A | Calycosin | Formononetin | Glycitein | Irigenin | 5-O-methylgenistein | Pratensein | Prunetin | Psi-tectorigenin | Retusin | TectorigeninGlycosides Prenylated isoflavones Bidwillol A | Derrubone | Luteone | WighteonePyranoisoflavones Alpinumisoflavone | Barbigerone | Di-O-methylalpinumisoflavone | 4'-methyl-alpinumisoflavoneMisc RotenoidsSynthetic Categories:
Wikimedia Foundation. 2010.
