- Trimethylsilyl cyanide
-
Trimethylsilyl cyanide 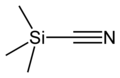
 trimethylsilylformonitrileOther namesCyanotrimethylsilane; TMS cyanide; Trimethylsilylnitrile; Trimethylsilanecarbonitrile' Trimethylsilylcarbonitrile
trimethylsilylformonitrileOther namesCyanotrimethylsilane; TMS cyanide; Trimethylsilylnitrile; Trimethylsilanecarbonitrile' TrimethylsilylcarbonitrileIdentifiers Abbreviations TMSCN CAS number 7677-24-9 
PubChem 82115 ChemSpider 74110 
Jmol-3D images Image 1 - C[Si](C)(C)C#N
Properties Molecular formula C4H9NSi Molar mass 99.21 g mol−1 Density 0.793 g/mL at 20 °C Melting point 8-11 °C, 281-284 K, 46-52 °F
Boiling point 114-117 °C, 387-390 K, 237-243 °F
Solubility in water organic solvents Solubility reacts with water Refractive index (nD) 1.392 Hazards R-phrases R11 R26/27/28 R29 S-phrases S16 S36/37/39 S45 Flash point 1 °C (34 °F)  cyanide (verify) (what is:
cyanide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:[1]
- LiCN + (CH3)3SiCl → (CH3)3SiCN + LiCl
In its principal reaction, it adds across carbon-oxygen double bonds, for example in an aldehyde:
- RCHO + (CH3)3SiCN → RCH(CN)OSi(CH3)3
The product is an O-silylated cyanohydrin.
One use of this reagent is to convert pyridine-N-oxides into 2-cyanopyridine. This transformation is best done in dichloromethane solution using dimethyl carbamoyl chloride as the activating electrophile. It is possible to use benzoyl chloride but the yields and regioselectivity of the addition of the cyano group are lower.
Safety
Trimethylsilyl cyanide is treated with care since it hydrolyzes to give hydrogen cyanide:
- 2 (CH3)3SiCN + H2O → (CH3)3SiOSi(CH3)3 + 2 HCN
References
- ^ Livinghouse, T. (1990), "Trimethylsilyl Cyanide: Cyanosilation of p-Benzoquinone", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV7P0517; Coll. Vol. 7: 517
Categories:- Nitriles
- Silanes
Wikimedia Foundation. 2010.
