- Pyrylium salt
-
Pyrylium 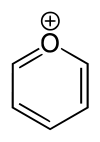
 Pyrylium
PyryliumIdentifiers PubChem 9548819 Jmol-3D images Image 1 - C1=CC=[O+]C=C1
Properties Molecular formula C5H5O+ Molar mass 81.09 g/mol  salt (verify) (what is:
salt (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references A pyrylium salt is a salt containing a pyrylium cation or a derivative of it.[1] [2] [3] [4] [5] [6] The pyrylium cation is a conjugated 6-membered carbon ring system with one carbon atom replaced by a positively charged oxygen atom. It is, like benzene, an aromatic compound.
A pyrylium may be any derivative of the pyrylium cation.Contents
Chemical properties
The carbon to oxygen double bond is also an oxonium ion, which due to aromatic stabilization is much less reactive than ordinary oxonium ions. However, the parent compound pyrylium is unstable in neutral water like oxonium salts. Pyrylium cations react with nucleophiles in the 2,4 and 6 positions and may result in ring-opening reactions. The high electronegativity of the oxygen results in the strongest single perturbation of one heteroatom in a six-membered ring.
Being aromatic, pyrylium salts are easily formed from simple starting materials, and their reactivity toward nucleophiles makes them useful materials for obtaining other compounds with stronger aromatic character. Thus, pyrylium salts afford pyridines with ammonia [7], pyridinium salts with primary amines, pyridine-N-oxides with hydroxylamine, phosphabenzenes with phosphine derivatives, thiopyrylium salts with hydrogen sulfide, and benzene derivatives with acetonitrile or nitromethane.
Synthesis
Pyrylium salts with aromatic substituents such 2,4,6-triphenylpyrylium tetrafluoroborate can be obtained from two moles of acetophenone and one mole of benzaldehyde in the presence of tetrafluoroboric acid and an oxidizing agent (Dilthey synthesis). For pyrylium salts with alkyl substituents such as 2,4,6-trimethylpyrylium salts, the best method uses the Balaban-Nenitzescu-Praill synthesis from tertiary butanol and acetic anhydride in the presence of tetrafluoroboric, perchloric, or trifluoromethanesulfonic acids. [8] [9] 2,4,6-Triphenylpyrylium salts are converted by bases into stable a 1,5-enedione (pseudobase) but 2,4,6-trimethylpyrylium salts on treatment with hot alkali hydroxides afford an unstable pseudobase that undergoes an intramolecular condensation yielding 3,5-dimethylphenol. In warm deuterium oxide, 2,4,6-trimethylpyrylium salts undergo isotopic exchange of 4-methyl hydrogens faster than for the 2- and 6-methyl groups, allowing the synthesis of regioselectively deuterated compounds.
Pyrones
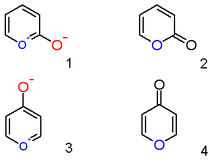
A pyrylium cation with a hydroxyl anion substituent in the 2-position is not the zwitterionic aromatic compound (1) but a neutral unsaturated lactone or an α-pyrone or pyran-2-one (2). Important representatives of this class are the coumarins. Likewise a 4-hydroxyl pyrylium compound is a φ-pyrone or pyran-4-one (4) to which group belongs a compound such like maltol.
Chemical properties
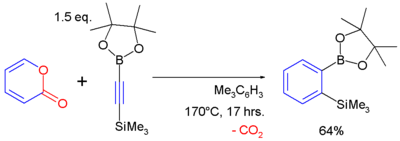
2-Pyrones are known to react with alkynes in a Diels-Alder reaction to form arene compounds with expulsion of carbon dioxide, for example:[10]
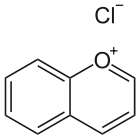
Chromenylium ion
The benzo-fused pyrylium ion is also called benzopyrilium or according to IUPAC chromenylium ion (Formula: C9H7O, molar mass: 131.15 g/mol, exact mass: 131.04968983). This is the charged version of 1-benzopyran or chromene (IUPAC).
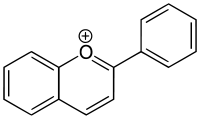
Flavylium ion
In biology, the ion 2-phenylchromenylium is referred to as flavylium. A class of flavylium derived compounds are anthocyanidins and anthocyanins, i.e., pigments that are responsible for the colors of many flowers.
See also
- 6-Membered aromatic rings with one carbon replaced by another group: borabenzene, benzene, silabenzene, germanabenzene, stannabenzene, pyridine, phosphorine
- Pyrans are pyrones lacking the ketone group.
References
- ^ Heterocyclic Chemistry, T. L. Gilchrist, ISBN 0-582-27843-0
- ^ A.T. Balaban, W. Schroth and G. Fischer, “Pyrylium Salts. I. Synthesis”, in Advances in Heterocyclic Chemistry, (eds. A. R. Katritzky and A. J. Boulton), Academic Press, New York, 1969, vol. 10, pp. 241–326.
- ^ A.T. Balaban, A. Dinculescu, G.N. Dorofeenko, G.W. Fischer, A.V. Koblik, V.V. Mezheritskii and W. Schroth, “Pyrylium Salts. Syntheses, Reactions and Physical Properties”, Advances in Heterocyclic Chemistry, Suppl. Vol. 2 (A. R. Katritzky, Ed.), Academic Press, New York, 1982.
- ^ A.T. Balaban, “The pyrylium cation as a synthon in organic chemistry”, in “New Trends in Heterocyclic Chemistry”, (eds. R.B. Mitra, N.R. Ayyangar, V.N Gogte, R.M. Acheson and N. Cromwell), Elsevier, Amsterdam, 1979, pp. 79–111.
- ^ A.T. Balaban, “Pyrylium salts as useful synthons”, in “Organic Synthesis : Modern Trends” (Proc. 6th IUPAC Internat. Symp. on Organic Synthesis, Moscow), (ed. O. Chizov), Blackwell, Oxford, 1987, pp. 263–274.
- ^ T.S. Balaban and A.T. Balaban, “Pyrylium Salts”. In “Science of Synthesis; Houben-Weyl Methods of Molecular Transformations”, Georg Thieme Verlag, Stuttgart, 2003, Vol 14, pp. 11–200.
- ^ 2,6-Di-tert-butyl-4-methylpyridine Organic Syntheses, Coll. Vol. 7, p.144 (1990); Vol. 60, p.34 (1981). Link
- ^ A.T. Balaban and A.J. Boulton, Org. Synth., Coll. Vol.5, 1112-1113 (1973): 2,4,6-Trimethyl-pyrylium tetrafluoroborate.Link
- ^ A.T. Balaban and A.J. Boulton, Org. Synth., Coll. Vol.5, 1114-1116 (1973): 2,4,6-Trimethyl-pyrylium trifluoromethanesulfonate. Link
- ^ An alkynylboronic ester cycloaddition route to functionalised aromatic boronic esters Patrick M. Delaney, Jane E. Moore and Joseph P. A. Harrity Chem. Commun., 2006, 3323 - 3325, doi:10.1039/b607322k
Categories:- Oxygen heterocycles
Wikimedia Foundation. 2010.