- Oxonium ion
-
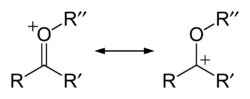
The oxonium ion in chemistry is any oxygen cation with three bonds. [1] The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g. R-C=O+-R' which forms a resonance structure with the fully fledged carbocation R-C+-O-R' and is therefore especially stable:
Stable alkyloxonium salts exist; they are extensively used as alkylating agents. For example, triethyloxonium tetrafluoroborate (Et3O+)(BF−
4) is a white crystalline solid. It is a powerful ethylating agent. It can be used, for example, to produce ethyl esters when the conditions of traditional Fischer esterification are unsuitable.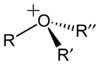
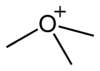
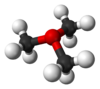
general pyramidal
oxonium ionskeletal formula of the
trimethyloxonium cationball-and-stick model
of trimethyloxoniumspace-filling model
of trimethyloxoniumOther hydrocarbon oxonium ions are formed by protonation or alkylation of alcohols or ethers (R-C-O+-R1R2). In acidic media, the oxonium functional group produced by protonating an alcohol can be a leaving group in the E2 elimination reaction, because when it receives an electron, it becomes a water molecule. The product is an alkene. Extreme acidity, heat and dehydrating conditions are usually required.
Oxatriquinane and oxatriquinacene are unusually stable oxonium ions, first described in 2008. Oxatriquinane does not react with boiling water or with alcohols, thiols, halide ions, or amines, although it does react with stronger nucleophiles such as hydroxide, cyanide, and azide.
See also
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
Categories:- Oxycations
Wikimedia Foundation. 2010.