- Oxatriquinane
-
Oxatriquinane 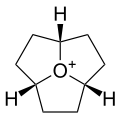
 (2as,4as,6as)-Octahydro-1H-2a1-oxacyclopenta[cd]pentalen-2a1-ium
(2as,4as,6as)-Octahydro-1H-2a1-oxacyclopenta[cd]pentalen-2a1-iumIdentifiers CAS number 1056549-35-9 
Jmol-3D images Image 1 - [H][C@@]12[O+]3[C@@](CC2)([H])CC[C@]3([H])CC1
Properties Molecular formula C9H15O Molar mass 139.21 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
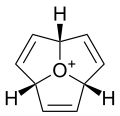
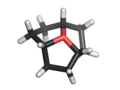
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oxatriquinane is an alkyl oxonium ion with formula C9H15O+, remarkable for being stable in aqueous solution. It has a cyclononane backbone, with the trivalent oxygen connected to carbons 1,4, and 7, forming three fused pentagonal cycles.
Oxonium ions normally are strong alkylating agents and are only observed in solution as reactive intermediates or under extreme conditions. Oxatriquinane does not react with boiling water or with alcohols, thiols, halide ions, or amines, although it does react with stronger nucleophiles such as hydroxide, cyanide, and azide. Oxatriquinane was first described in 2008, and was obtained after a five-step synthesis starting from cyclononatriene.[1][2][3] The same paper also described oxatriquinacene, the tri-unsaturated analog, which is of interest as a possible precursor to oxaacepentalene, a neutral aromatic species.
References
- ^ Mark Mascal, Nema Hafezi, Nabin K. Meher, and James C. Fettinger (2008). "Oxatriquinane and Oxatriquinacene: Extraordinary Oxonium Ions". Journal of the American Chemical Society 130 (41): 13532–13533. doi:10.1021/ja805686u. PMID 18798616.
- ^ Rachel Petkewich (September 29, 2008). "Taming Alkyl Oxonium Ions: Fused tricyclic structure stabilizes famously reactive alkylating agents". Chemical and Engineering News 86 (39): 10. doi:10.1021/cen-v086n039.p010. http://pubs.acs.org/cen/news/86/i39/8639notw6.html.
- ^ Tim Reid (3 October 2008). "Oxonium ions: Ring of stability". Nature Chemistry. doi:10.1038/nchem.70.
Categories:- Oxycations
- Oxygen heterocycles
Wikimedia Foundation. 2010.