- Maternal effect
-
- This article concerns the legitimate scientific concept of genes that are expressed only when carried by the female parent. It is not to be confused with the generally discredited theory of maternal impression.
A maternal effect is a situation where the phenotype of an organism is determined not only by the environment it experiences and its genotype, but also by the environment and phenotype of its mother. In genetics, maternal effects occur when an organism shows the phenotype expected from the genotype of the mother, irrespective of its own genotype, often due to the mother supplying mRNA or proteins to the egg. Maternal effects can also be caused by the maternal environment independent of genotype, sometimes controlling the size, sex, or behaviour of the offspring. It has been proposed that maternal effects are important for the evolution of adaptive responses to environmental heterogeneity.
Contents
Maternal effects in genetics
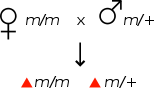
In genetics, a maternal effect occurs when the phenotype of an organism is determined by the genotype of its mother.[1] For example, if a mutation is maternal effect recessive, then a female homozygous for the mutation may appear phenotypically normal, however her offspring will show the mutant phenotype, even if they are heterozygous for the mutation.
Maternal effect All offspring show the wild-type phenotype All offspring show the mutant phenotype Genetic crosses involving a maternal effect recessive mutation, m. The maternal genotype determines the phenotype of the offspring. Maternal effects often occur because the mother supplies a particular mRNA or protein to the oocyte, hence the maternal genome determines whether the molecule is functional. Maternal supply of mRNAs to the early embryo is important, as in many organisms the embryo is initially transcriptionally inactive.[2] Because of the inheritance pattern of maternal effect mutations, special genetic screens are required to identify them. These typically involve examining the phenotype of the organisms one generation later than in a conventional (zygotic) screen, as their mothers will be potentially homozygous for maternal effect mutations that arise.[3][4]
Example: maternal effect genes in Drosophila early embryogenesis
A Drosophila melanogaster oocyte develops in an egg chamber in close association with a set of cells called nurse cells. Both the oocyte and the nurse cells are descended from a single germline stem cell, however cytokinesis is incomplete in these cell divisions, and the cytoplasm of the nurse cells and the oocyte is connected by structures known as ring canals.[5] Only the oocyte undergoes meiosis and contributes DNA to the next generation.
Many maternal effect Drosophila mutants have been found that affect the early steps in embryogenesis such as axis determination, including bicoid, dorsal, gurken and oskar.[6][7][8] For example, embryos from homozygous bicoid mothers fail to produce head and thorax structures.
Once the gene that is disrupted in the bicoid mutant was identified, it was shown that bicoid mRNA is transcribed in the nurse cells and then relocalized to the oocyte.[9] Other maternal effect mutants either affect products that are similarly produced in the nurse cells and act in the oocyte, or parts of the transportation machinery that are required for this relocalization.[10] Since these genes are expressed in the (maternal) nurse cells and not in the oocyte or fertilised embryo, the maternal genotype determines whether they can function.
Environmental maternal effects
The environment or condition of the mother can also in some situations influence the phenotype of her offspring, independent of the offspring's genotype.
Paternal effect genes
In contrast, a paternal effect is when a phenotype results from the genotype of the father, rather than the genotype of the individual.[11] The genes responsible for these effects are components of sperm that are involved in fertilization and early development.[12] An example of a paternal-effect gene is the ms(3)sneaky in Drosophila, males with a mutant allele of this gene produce sperm that are able to fertilize an egg, but the snky-inseminated eggs do not develop normally. However, females with this mutation produce eggs that undergo normal development when fertilized.[13]
See also
References
- ^ Griffiths, Anthony J. F. (1999). An Introduction to genetic analysis. New York: W. H. Freeman. ISBN 071673771X. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=maternal,effect&rid=iga.section.3739#3740.
- ^ Schier AF (April 2007). "The maternal-zygotic transition: death and birth of RNAs". Science 316 (5823): 406–7. Bibcode 2007Sci...316..406S. doi:10.1126/science.1140693. PMID 17446392.
- ^ Jorgensen EM, Mango SE (May 2002). "The art and design of genetic screens: Caenorhabditis elegans". Nat. Rev. Genet. 3 (5): 356–69. doi:10.1038/nrg794. PMID 11988761.
- ^ St Johnston D (March 2002). "The art and design of genetic screens: Drosophila melanogaster". Nat. Rev. Genet. 3 (3): 176–88. doi:10.1038/nrg751. PMID 11972155.
- ^ Bastock R, St Johnston D (December 2008). "Drosophila oogenesis". Curr. Biol. 18 (23): R1082–7. doi:10.1016/j.cub.2008.09.011. PMID 19081037.
- ^ Nüsslein-Volhard C, Lohs-Schardin M, Sander K, Cremer C (January 1980). "A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila". Nature 283 (5746): 474–6. doi:10.1038/283474a0. PMID 6766208.
- ^ Schüpbach T, Wieschaus E (February 1986). "Germline autonomy of maternal-effect mutations altering the embryonic body pattern of Drosophila". Dev. Biol. 113 (2): 443–8. doi:10.1016/0012-1606(86)90179-X. PMID 3081391.
- ^ Nüsslein-Volhard C, Frohnhöfer HG, Lehmann R (December 1987). "Determination of anteroposterior polarity in Drosophila". Science 238 (4834): 1675–81. doi:10.1126/science.3686007. PMID 3686007.
- ^ Berleth T, Burri M, Thoma G, et al. (June 1988). "The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo". EMBO J. 7 (6): 1749–56. PMC 457163. PMID 2901954. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=457163.
- ^ Ephrussi A, St Johnston D (January 2004). "Seeing is believing: the Bicoid morphogen gradient matures". Cell 116 (2): 143–52. doi:10.1016/S0092-8674(04)00037-6. PMID 14744427.
- ^ Yasuda GK, Schubiger G, Wakimoto BT (1 May 1995). "Genetic characterization of ms (3) K81, a paternal effect gene of Drosophila melanogaster". Genetics 140 (1): 219–29. PMC 1206549. PMID 7635287. http://www.genetics.org/cgi/reprint/140/1/219.
- ^ Fitch KR, Yasuda GK, Owens KN, Wakimoto BT (1998). "Paternal effects in Drosophila: implications for mechanisms of early development". Curr. Top. Dev. Biol. 38: 1–34. doi:10.1016/S0070-2153(08)60243-4. PMID 9399075.
- ^ Fitch KR, Wakimoto BT (1998). "The paternal effect gene ms(3)sneaky is required for sperm activation and the initiation of embryogenesis in Drosophila melanogaster". Dev. Biol. 197 (2): 270–82. doi:10.1006/dbio.1997.8852. PMID 9630751.
The development of phenotype Key concepts Genetic architecture Dominance relationship · Epistasis · Polygenic inheritance · Pleiotropy · Plasticity · Canalisation · Fitness landscape · Transgressive phenotypeNon-genetic influences Developmental architecture Evolution of genetic systems Influential figures Debates List of evolutionary biology topics
Wikimedia Foundation. 2010.