- Tetrahydropyran
-
Tetrahydropyran 
 OxaneOther namesTetrahydropyran,
OxaneOther namesTetrahydropyran,
OxacyclohexaneIdentifiers CAS number 142-68-7 
PubChem 8894 ChemSpider 8554 
UNII V06I3ILG6B 
DrugBank DB02412 KEGG C15345 
ChEBI CHEBI:46941 
Jmol-3D images Image 1 - O1CCCCC1
Properties Molecular formula C5H10O Molar mass 86.13 g/mol Density 0.880 g/cm3 Melting point -45 °C, 228 K, -49 °F
Boiling point 88 °C, 361 K, 190 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tetrahydropyran is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. The compound is a colourless volatile liquid, but is obscure. Derivatives of tetrahydropyran are, however, more common. Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and dihydropyran are common intermediates in organic synthesis. Furthermore, tetrahydropyran ring system, i.e. a five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.
Contents
Preparation
One classic procedure for the organic synthesis of tetrahydropyran is by hydrogenation with Raney nickel of dihydropyran.[1]
Reactions
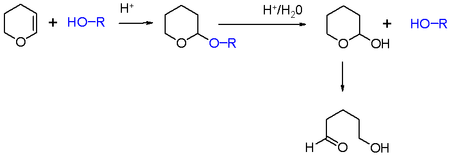
In organic synthesis, the 2-tetrahydropyranyl group is used as a protecting group for alcohols.[2][3] Reaction of the alcohol with dihydropyran forms a tetrahydropyranyl ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored readily by acidic hydrolysis with formation of 5-hydroxypentanal.
See also
- Tetrahydrofuran (THF)
- Pyran
References
- ^ D. W. Andrus; John R. Johnson (1955), "Tetrahydropyran", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0794; Coll. Vol. 3: 794
- ^ R. A. Earl L. B. Townsend (1990), "Methyl 4-Hydroxy-2-butynoate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0334; Coll. Vol. 7: 334
- ^ Arthur F. Kluge (1990), "Diethyl [(2-Tetrahydropyranyloxy)methyl]phosphonate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0160; Coll. Vol. 7: 160
Categories:- Protecting groups
- Ether solvents
- Tetrahydropyrans
Wikimedia Foundation. 2010.